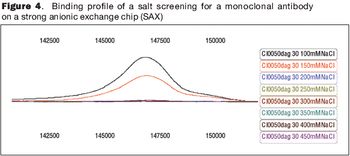
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.

The future of therapeutic MAbs lies in the development of economically feasible downstream processes.
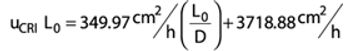
Robust packing procedures can improve process performance and increase resin lifetime.

A close-up look at Pfizer's biotherapeutics plant in Shanbally, Ireland.
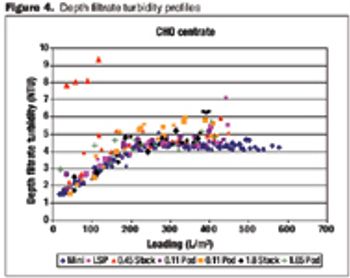
Data on the performance and variability of different formats.
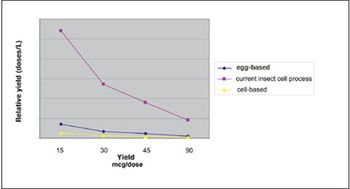
With virus-based production, vaccines can be available in 10-12 weeks.
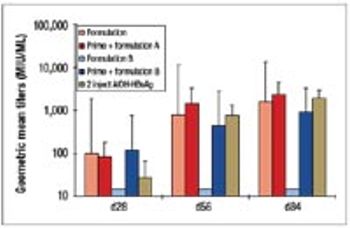
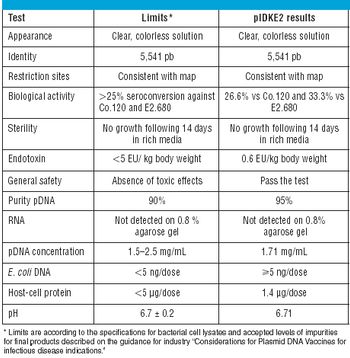
The disadvantages of the traditional vaccine regime (prime plus boost) have spurred the development of single-shot vaccines. This article describes the development and manufacture of a prototype single-shot vaccine that uses microspheres made from cross-linked modified dextran polymers for controlled release of the antigen.
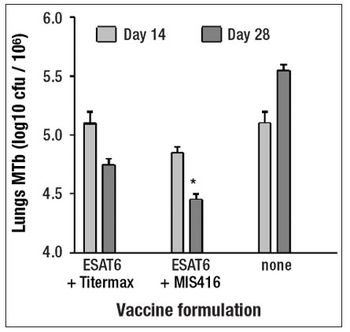
This article discusses the potential of MIS416 adjuvant, a vaccine adjuvant and immunogen co-delivery system, to provide adequate immunostimulation to overcome host factors that may limit the success of therapeutic vaccines.

Understanding the relationship between the process and CQAs.


The development of a skilled labor force is essential for an expanding biopharmaceutical industry.
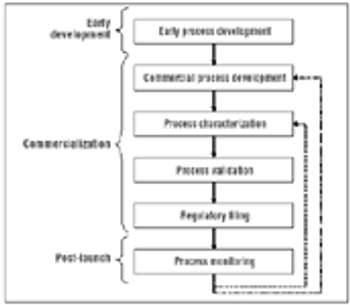
Using multivariate experiments to define acceptable ranges.
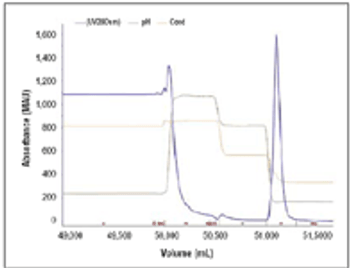
Results from a process developed for a commercial antibody.
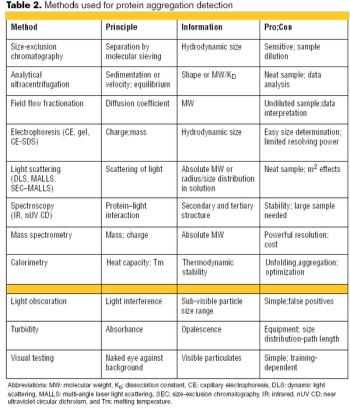
FDA perspectives on specs and effective control strategies.
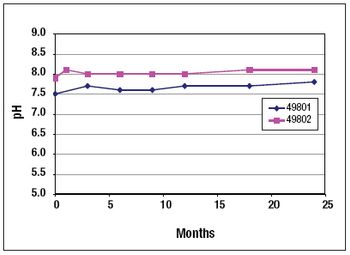
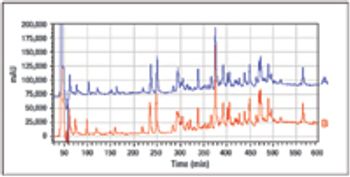
NeisVac-C is a polysaccharide-protein conjugate vaccine for use against Neisseria meningitidis serogroup C infection. The Phase 1 clinical formulation consisted of physiological saline, thimerosal, and aluminum hydroxide. The long-term stability data for both the PBS and saline formulations are presented in this article.

Interview with Roger Lias, president and group commercial director at Eden Biodesign, Inc.
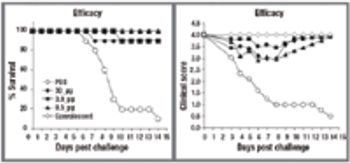
This article discusses the production process of the major influenza antigen, hemagglutinin (HA), by rDNA methods in E. coli.

Interview with Magda Marquet, co-CEO and co-president of Althea Technologies
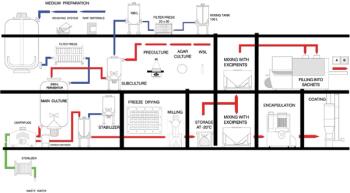
There are a number of specific characteristics to be considered when developing and manufacturing live bacterial vaccines.
The Center for Molecular Immunology (Havana, Cuba) has been working on a novel cancer immunotherapy targeting the epidermal growth factor (EGF). The vaccine is composed of a chemical conjugate of EGF and a carrier protein (rP64k), designed to trigger an anti-EGF antibody response. The results of studies of molecular characterization, immunogenic activity, and clinical data are presented here.

To succeed in a pandemic, the industry must forge a preparedness plan to ensure adequate vaccines.

The year 2007 witnessed the approval of fifteen biopharmaceuticals in the United States and European Union.

An analysis of current and upcoming industry challenges.

To achieve the right balance between disposable and reuseable options, companies must consider important technical and economic factors.

How to implement a risk-based approach to eliminating viruses.