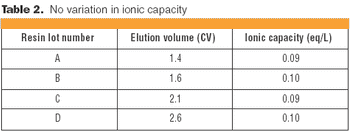
Understanding the impact on process performance.

Understanding the impact on process performance.

In the context of process validation, the confirmation of a belief must be checked repeatedly, throughout the product lifecycle.
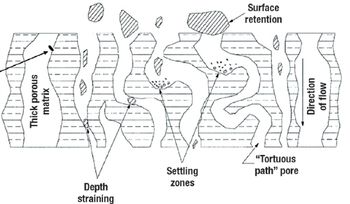
A comparison of primary harvest techniques.

The book is a useful, comprehensive, and truly an excellent reference source of biopharmaceutical information.

Disposable technologies that mimic the conventional stainless-steel bioreactor will be most readily adopted

A case study investigated the root cause of failures in sterile filtration by evaluating the effects and interactions of four operating parameters.
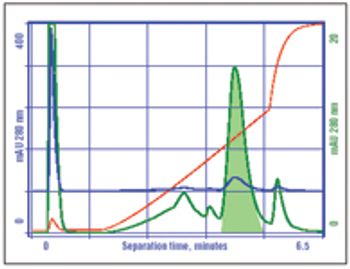
Manufacturing challenges surround the use of IgM monoclonal antibodies, but these can be overcome with current technology.
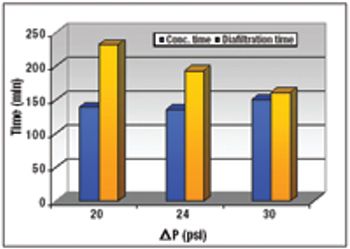
An alternative approach to traditional Protein A schemes is comparable in overall efficiency, product recovery, and quality.
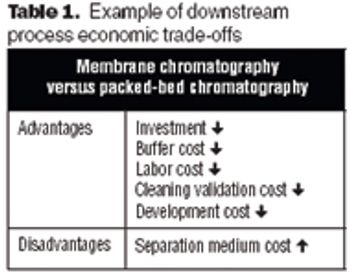
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.
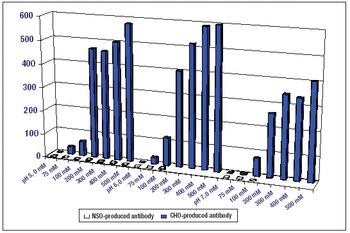
If certain engineering challenges can be addressed, precipitation may prove to be a valuable tool for antibody purification.

When platform processes are applied to fusion molecules, innovation and flexibility are needed.
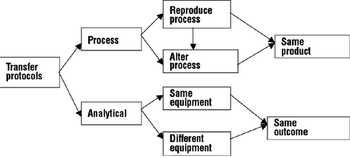
A comprehensive process and analytical transfer package can speed up your product's time to market and save costs.

How an electronics engineer led the first Indian company to carry out indigenous development of a recombinant vaccine.
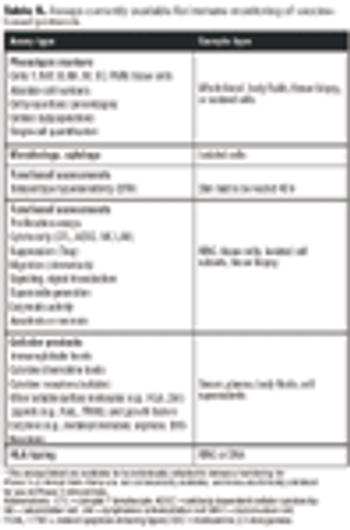
Emerging therapies pose challenges for standardizing QC.

Two case studies illustrate a systematic approach.

The use of disposables has changed significantly in the biopharmaceutical industry.

The replacement of a IgG ELISA assay with an SPR-based test can shorten the production workflow by one day.
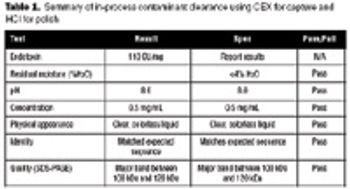
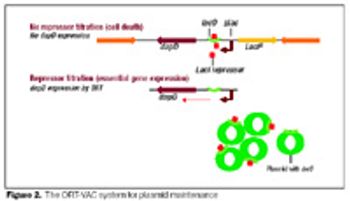
The recombinant approach lends itself to the production of an inexpensive and effective vaccine at large scale.

Potential interference with maternally-derived antibodies makes most vaccines less effective in the neonate.
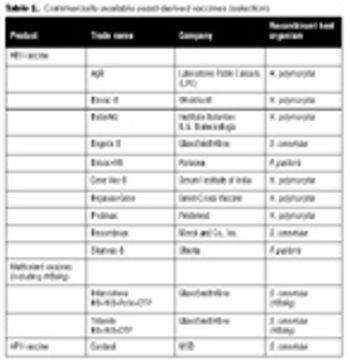
Human HBV can be grouped into eight genotypes, which differ by at least 8%.
Microstructured transdermal systems can deliver a vaccine in close proximity to the antigen-presenting cells in the epidermis.

Design space concepts are key to a successful technology transfer.

How the authors used design of experiments and quality by design principles to develop a hydrophobic interaction chromatography step.


The challenge is not in coming up with a list of activities to discard, but in finding a feasible way to stop doing them.