
Patients receiving palliative care in hospices, hospitals, and other settings can benefit from a subcutaneous injection of morphine with Hylenex recombinant (hyaluronidase human injection).

Patients receiving palliative care in hospices, hospitals, and other settings can benefit from a subcutaneous injection of morphine with Hylenex recombinant (hyaluronidase human injection).

The biopharmaceutical industry has gained a lot of experience in monitoring glycosylation, but still has a lot to learn about the structure–function relationship.

The United States Pharmacopeia (USP, Rockville, MD, www.usp.org) and the UK's National Institute for Biological Standards and Control (NIBSC, Hertfordshire, UK, www.nibsc.ac.uk) are seeking participants in a study of analytical methods used by the industry to characterize and quantify oligosaccharides.

Cell Therapeutics, Inc. (CTI, Seattle, WA, www.ctiseattle.com), has formed a new spin-off company, Aequus BioPharma, Inc., to develop a novel process to extend the half-life of proteins.
To shorten time to market for new therapeutic proteins, new and fast methods, such as high throughput screening, are needed to speed up downstream processing. The platform technology discussed in this article includes a structural approach that can be used as a general procedure to purify therapeutic proteins. The approach starts with ligand screening and selection-on-a-chip, with the Surface Enhanced Laser Desorption Ionization–Time of Flight (SELDI–TOF) mass spectrometer system. Next, resin screening and supplier selection are performed using robotics, followed by scouting studies under dynamic conditions to select the best resin. Finally, optimization studies of critical parameters are carried out with statistical design approaches (design of experiments). A few examples are presented to explain the platform approach for purification development in more detail.
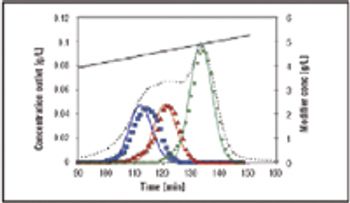
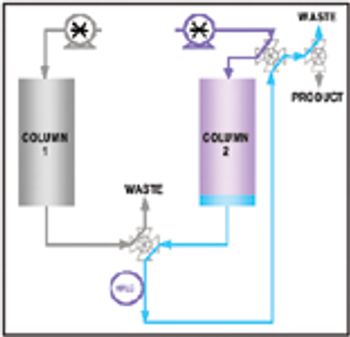
This article presents the multicolumn countercurrent solvent gradient purification (MCSGP) process, which uses three chromatographic columns, and incorporates the principle of countercurrent operation and the possibility of using solvent gradients. A MCSGP prototype has been built using commercial chromatographic equipment. The application of this prototype for purifying a MAb from a clarified cell culture supernatant using only a commercial, preparative cation exchange resin shows that the MCSGP process can result in purities and yields comparable to those of purification using Protein A.

This article explores the development of process chromatography. Process chromatography was first applied to the removal of low molecular weight solutes from whey by gel filtration about 50 years ago. An analytical method using size exclusion chromatography was scaled up for insulin production in the 1970s, when ion exchange became a viable technology for the same application. Ion exchange was adopted as the industry workhorse as robust resins became available and formed the backbone of chromatographic processing of blood plasma fractionation in alternatives to and extensions of ethanol precipitation.

These articles encapsulate the past, present, and possible future of process-scale chromatography in biopharmaceutical production.
This article discusses how on-line high-performance liquid chromatography (HPLC) can measure product purity in the column eluent stream in near–real time. These data can then enable the automation and control of a purification column operation, thus reducing product variability, shortening process cycle time, and increasing yield. An example application demonstrates how on-line HPLC is used as a process analytical technology to ensure the process can accommodate variability in the separation while ensuring the product meets its critical quality attributes.
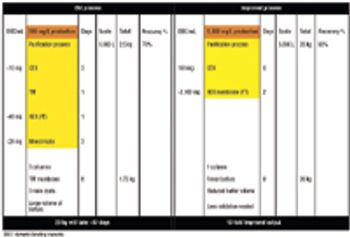
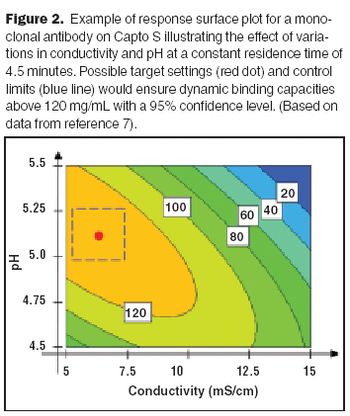
Affinity purification schemes for antibody production have certain limitations keeping up with cell culture expression levels as they reach and exceed 10 g/L. New downstream purification processes are based on low cost, long lasting, and high binding (40–100 mg/mL) cation exchange resins.

The HSV-1 and HVP-2 titers were determined by the inoculation of test solutions into Vero cell cultures and calculated using the Reed M?ench method.

Conducting an analysis of the 4 Ms-man, machine, methods, and materials-enables companies to identify the true root causes of deviations.
The many benefits of disposable technologies, such as significant savings in time, labor and capital, as well as ease of scalability and flexibility, have led to the growing trend of adopting disposable technologies in bioprocess manufacturing processes.
In recent years, disposable membrane chromatography has gained acceptance as a robust device for large-scale processing.
Biopharmaceutical processes typically require a significant investment in equipment-often a substantial obstacle for start-up companies. The risk of drug development failure is often high, further limiting access to the required capital. Flexibility and lower capital outlays are required not only by start-up companies, but also by research organizations with multiple product lines and by companies requiring quick capacity increases. Disposable technologies offer the highest potential for these companies to meet their business requirements. With lower capital requirements and increased flexibility, disposables are an important part of these companies' risk management strategy.

Filtration systems exemplify disposable technologies that can be presterilized.

Increased resin stability can extend the number of cleaning cycles that can be performed in situ.

The number of biotechnology-based human therapeutic products in the late-stage pipeline, and the average cost to commercialize a biotech product, have steadily increased. This has required biotech companies to use economic analysis as a tool during process development and for making decisions about process design. Process development efforts now aim to create processes that are economical, as well as optimal and robust.

If a company wants to reduce costs, it should consider outsourcing some manufacturing and analytical testing to low-cost sites.

This article shows how Probabilistic Tolerance Intervals of the form, "We are 99% confident that 99% of the measurements will fall within the calculated tolerance limits" can be used to set acceptance limits using production data that are approximately Normally distributed. If the production measurements are concentrations of residual compounds that are present in very low concentrations, it may be appropriate to set acceptance limits by fitting a Poisson or an Exponential Distribution.

We often assume we know what success looks like for our partner, but we never ask them, or take the time to write it down.
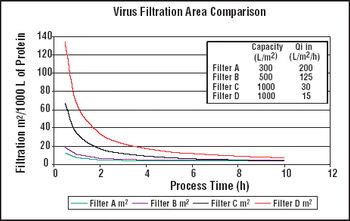
It is important to ensure that flow decay during processing is comparable to that observed during retention studies.

Formulations for pulmonary inhalation comprise spherical, porous particles that are 1–3 microns in diameter.

Established, fully validated methodology and SOPs are required prior to initiation of any training activities.