
Manufacturing, Solid Dosage Drugs
Latest News

Latest Videos
More News

Small-molecule therapeutics can also benefit from the implementation of PAT in the manufacturing process.

The partnership and the formation of the institute intend to bring together industry, academia, and regulators to tackle challenges and provide solutions for continuous manufacturing.

A $5.5 billion acquisition brings Capsugel’s oral dosage delivery capabilities to Lonza’s portfolio.
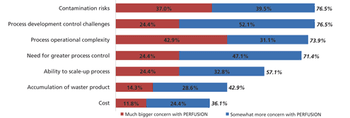
Although widespread adoption of continuous bioprocessing has been slow, some processes have been an exception.

Approval of breakthrough therapies requires expedited quality assessment.

High-purity low-endotoxin sugars improve robustness and stability of protein formulation and improve drug product quality.

Challenging molecules and markets are driving the development of new solutions for drug delivery.

A new study examines the oral delivery of protein drugs in plant cells and hypothesizes that the cold storage and transportation of biologics could someday be eliminated.

The Subcommittee for Advanced Manufacturing of the National Science and Technology Council highlights biopharmaceutical manufacturing as an emerging priority.

Demand is driving expansion and consolidation of formulation and clinical trial materials services.

The “next-generation” design for the pods will build on Pfizer’s existing modular prototype for oral solid-dose manufacturing.

The company presented a portfolio of new products during the meeting in Madrid.

The Pre-Connect Congress will explore pharma industry trends, such as mergers and acquisitions, the biologics market outlook, and innovation in drug delivery among others.

FDA issues draft guidance on dissolution testing for immediate-release solid oral dosage forms.

The authors explore the use of statistical experimental design and multivariate analysis to develop a drug substance formulation matrix.

The new service offering includes feasibility studies, process and analytical development, and scale-up from milligram to gram quantities required for pilot and subsequent commercial scale.

Will AbbVie stave off biosimilar competitors by following Teva’s model for Copaxone?

The new facility expands the company’s commercial manufacturing capability at its Bend, Ore. site.

Integrating advances in facility design can meet differing and emerging bioprocessing needs.

Ethylene vinyl acetate (EVA) drug-release technologies are explored.

The report highlights a need for greater third party certification to ensure GMP vigilance.

The facility, which includes state-of-the-art formulation, analytical and synthetic laboratories as well as a customer training center, will focus on bioavailability enhancement and oral dosage formulations.

Do we really have to choose between saving money and saving time?
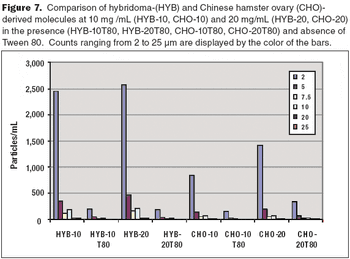
How to maintain product stability and prevent particulates.
