
The author discusses HTST pasteurization and UHT sterilization.

The author discusses HTST pasteurization and UHT sterilization.

Viruses in animal-derived starting materials could contaminate biopharmaceutical final product. A rigorous testing strategy and removal methods are reviewed.

Industry experts discuss the implementation of QbD and PAT tools in biopharmaceutical manufacturing.

The authors provide common misconceptions and key concepts behind reliability engineering.
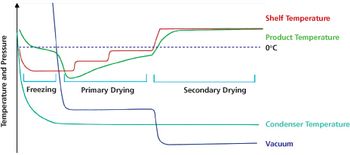
Optimized freeze-drying cycles can offer scientific and business advantages.

Aggregate formation is influenced by multiple aspects of the bioproduction process but can be mitigated by good process design and control.

What the industry's future holds and what needs to be done to get there.

NIBRT's Ray O'Connor provides an overview of aseptic processing.
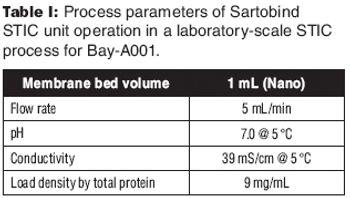
A new downstream purification platform using a salt-tolerant membrane adsorber.
The author examines the use of closures for products intended for injection.
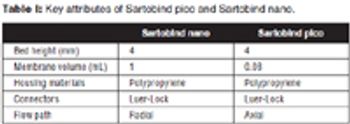
The authors describe the development of an ultra scale-down anion exchange membrane adsorber, and demonstrate scalability to larger-scale devices.

GE Healthcare Life Sciences' ReadyToProcess platform aims to streamline bioprocessing.
Insights on single-use systems implementation and exploitation in biopharmaceutical manufacturing and processing, based on a QbD approach.
This month, Jerold Martin of Pall Life Sciences takes a look at protein recovery through direct-flow microporous membrane filters over the past 25 years.

The author considers the types of tubing available to the industry and how to make an informed selection.

The European Medicines Agency has added granularity to its biosimilars approval pathway by releasing a guideline on mAbs.

NIBRT's Ian Nelligan on what to expect when starting a downstream process.

Michiel E. Ultee of Laureate Biopharmaceutical Services gives an update on 1988 article regarding virus testing and how the advance of monoclonal antibodies has changed processes.
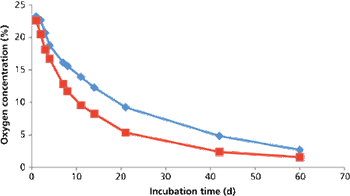
The authors describe a validation master plan for closed-vial filling technology.

Steven S. Kuwahara, PhD, principal consultant at GXP BioTechnology LLC, gives an update on "Engineering the Cell-System Interface."
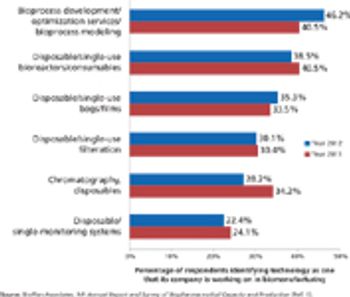
Industry wants more innovation, but can suppliers meet customers' needs?

Panayiotis P. Constantinides of Biopharmaceutical & Drug Delivery Consulting on growth of nanoparticle delivery systems.

A review of key industry shifts and promises for the future.

Industry experts discuss significant achievements. Plus: What's in store for the future.

This article discusses potential opportunities to improve the patient experience through formulation and delivery device technologies.