
Multiparticulates are increasingly used due to their flexibility in providing controlled-release, fixed-dose combinations, ease of taste-masking, and suitability for pediatric applications

Multiparticulates are increasingly used due to their flexibility in providing controlled-release, fixed-dose combinations, ease of taste-masking, and suitability for pediatric applications

The Pre-Connect Congress will explore pharma industry trends, such as mergers and acquisitions, the biologics market outlook, and innovation in drug delivery among others.

Industry experts spoke to BioPharm International about the key considerations in the development of a drug-delivery device for a biologic drug, the importance of human factors engineering, the advantages of prefilled syringes, and the challenges in the manufacture of these devices.

Recently published research demonstrates how nanoparticles can be used to overcome hurdles in localized drug delivery.

A team from Northwestern University has demonstrated the feasibility of topical delivery of small interfering RNA (siRNA).

Panayiotis P. Constantinides of Biopharmaceutical & Drug Delivery Consulting on growth of nanoparticle delivery systems.
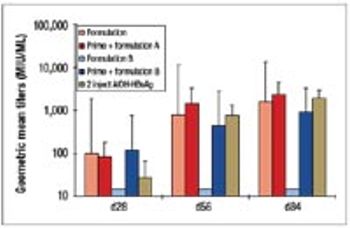
The disadvantages of the traditional vaccine regime (prime plus boost) have spurred the development of single-shot vaccines. This article describes the development and manufacture of a prototype single-shot vaccine that uses microspheres made from cross-linked modified dextran polymers for controlled release of the antigen.