
This article highlights 15 years of changes in biopharmaceutical manufacturing.

This article highlights 15 years of changes in biopharmaceutical manufacturing.

Effective communication between contract manufacturing organizations and pharmaceutical company clients relies on well-defined master service and quality agreements.

FDA’s focus on the quality culture and its request for quality metrics may ensure a successful company-CMO relationship.

New drug approval policies promote industry growth and injectable contract manufacturing opportunities in China.

Biopharmaceutical manufacturers continue to put quality at the forefront of their relationships.
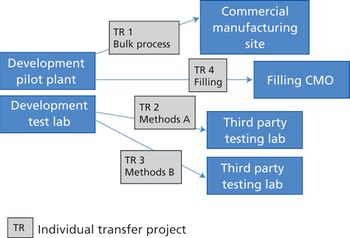
Careful planning, adequate staffing levels, and experienced project managers can help avoid pitfalls of transferring processes from one facility to another.

While stakeholders generally welcome improvements to quality initiatives, they are concerned with how the new requirements will be implemented for more complicated supply-chain models.

A well-constructed quality agreement can be an important tool to enable effective collaboration between owner and CMO.
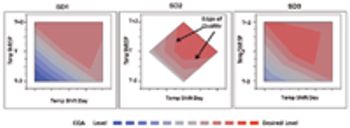
Traditional project decision-making vs. a QbD approach.
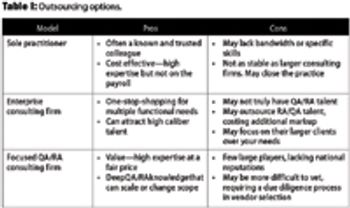
This article examines the options to best match needs and spending for quality and regulatory leadership.

Implementing quality by design makes the determination of quality metrics across CMOs and sponsors essential.

The contract provider needs to know as much as the NDA holder.

Contract organizations must have highly organized teams and plans to accommodate today's audits.

Rapid microbial screening provided by contract laboratories can save companies time and money.

An enterprise-wide quality management initiative is required to maintain supplier quality without sacrificing bottom-line objectives.

To stave off quality issues associated with production processes, a centralized and enterprise-wide quality management initiative must be enforced.

India is restructuring its regulation of biopharmaceuticals to help the country's industry compete internationally.

How the authors used design of experiments and quality by design principles to develop a hydrophobic interaction chromatography step.
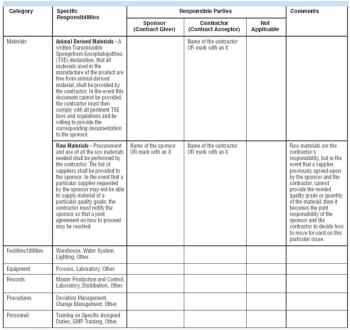
During the past several years in the pharmaceutical and biopharmaceutical industries, conflicts and misunderstandings have arisen between companies and their contractors. Too often, productive working relationships have crumbled, resulting in expensive production delays with companies and contractors squabbling over their roles and responsibilities. Such conflicts may have their roots in the lack of a sound quality agreement (QAG). QAGs that clearly delineate good manufacturing practice (GMP) responsibilities between a sponsor and a contractor can help companies and their contractors avoid certain conflicts.