Formulation and Drug Delivery, Biologic Drugs
Latest News
Latest Videos

More News

Non-parenteral alternatives for biologics remain a clinical imperative and a formidable challenge.

The industry is diversifying pipelines from traditional small-molecule drugs to embrace complex and exciting new modalities.

The spray-dried formulations for Ethris’ mRNA vaccine candidates will be developed at Lonza’s Bend, Ore., Center of Excellence in accordance with GMP standards.

The $94 million (€90 million) Series A funding will be used to support the development of the company’s pipeline of nCycles, oral macrocycle drugs that will be focused on validated biologic targets.

Lonza’s new tailored offering leverages the company’s bi-layer capsule manufacturing technology.

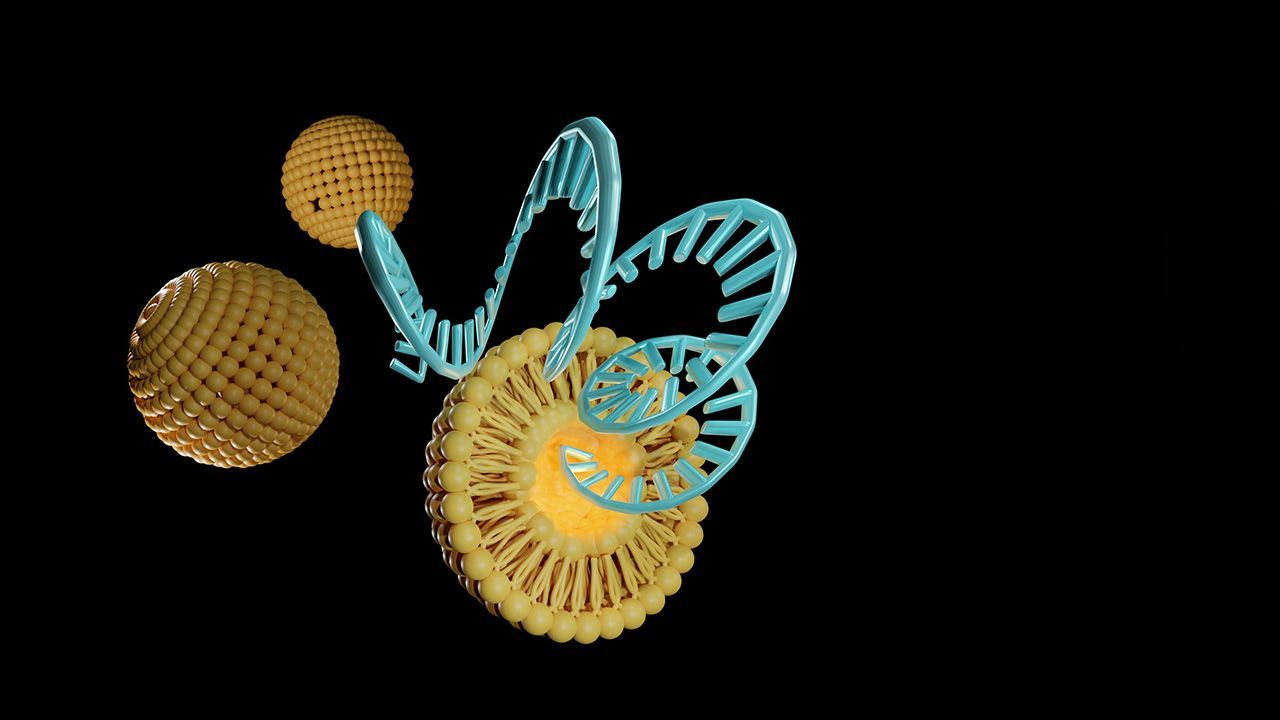
Under the collaboration, the companies will create and test circVec DNA–LNP formulations with an eye toward potential therapeutic applications.

This podcast explores the challenges of and the progress made so far by the biopharma industry toward alternative drug delivering methods for biologic drugs.

Alternative delivery methods for biologics continues to be explored that offer less invasive, less painful administration.

AAV and lentivirus both have pros and cons in their use for specific gene therapy applications.

Roche voluntarily recalled Susvimo’s ocular implant, insertion tool, and initial fill kit when test results did not satisfy company standards.

Swiss CDMO CordenPharma and Spain-based Certest will collaborate on the development of ionizable lipids for LNP formulations.
A comparison between polysorbates and HPβCD determines the better stabilizer for biologics formulation.

This article represents a comprehensive exploration about biopharmaceutical excipients, exploring their multifaceted classification, active role in drug formulation processes, inherent challenges, and upcoming advancements poised to revolutionize the drug formulation and its efficacy for patient welfare.
Despite slow growth over recent years, the CAR-T cell therapy space is expected to see considerable advancements in the near future.

Seagen will be responsible for conjugating these degraders to antibodies to make DACs and advancing these DAC drug candidates through preclinical and clinical development and commercialization.
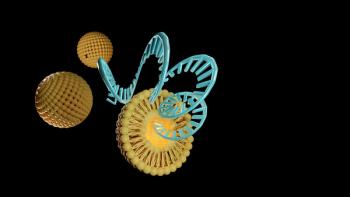
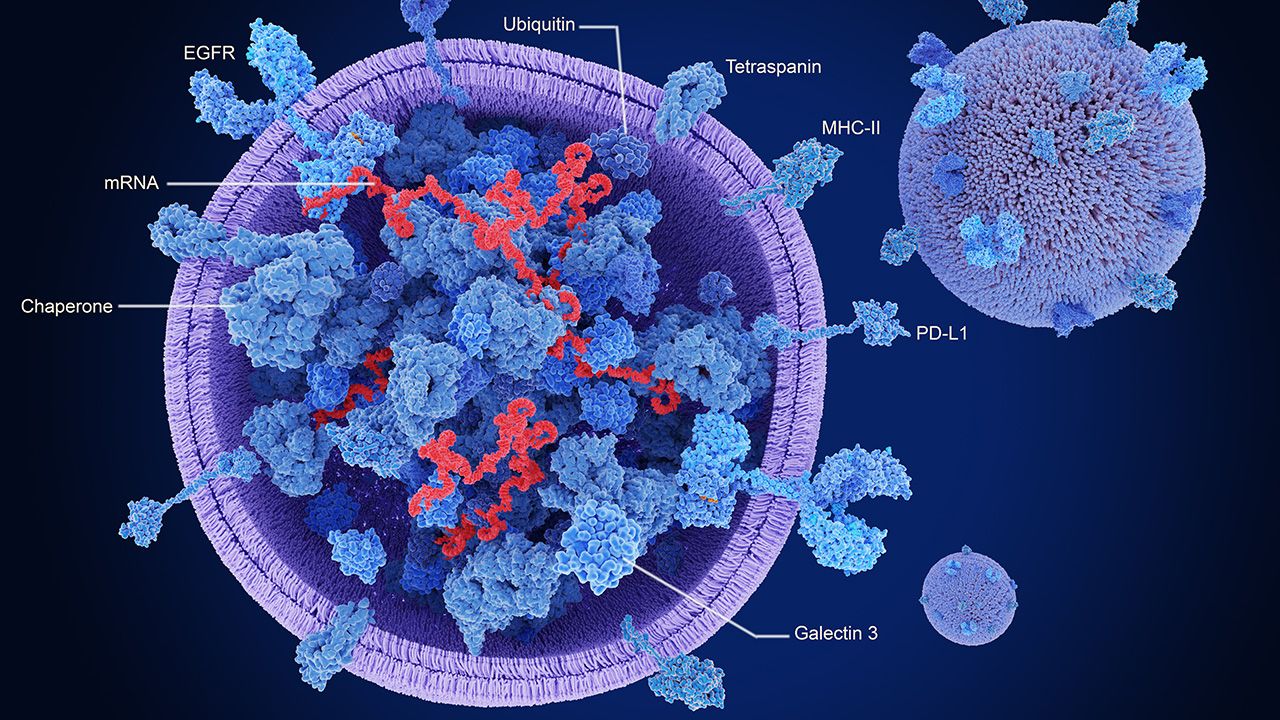
Nanoparticles offer the potential for a safer, more effective method of drug delivery to the patient.

There are solutions on the horizon that will help overcome the current bottlenecks and other challenges that are occurring in early drug development.

The integration of the binders into a routine lateral flow test will support the clinical and commercial development of rapid diagnostics for Alzheimer’s disease through nasal sampling.

Hanns-Christian Mahler and Andrea Allmendinger from ten23 health will discuss some key aspects of biologic drug development and manufacturing.

Outsourcing collaborations provide insight into the key drivers of market growth.

FDA’s Division of Applied Regulatory Science’s (DARS) computational toxicology and pharmacology research has been focused on the development of highly curated data sets and QSAR models for endpoints of regulatory interest.

Jaeger notes that the program is focused on addressing specific scientific questions that will produce immediate impacts on how CDER and FDA make drug approval decisions.

The guidance is intended to provide nonclinical, virology, and clinical considerations for mpox drug development programs, targeting on recommendations to support initiation of clinical trials.

This self-emulsifying system improves the miscibility and dispersibility of formulations in aqueous environments and may improve API solubility.

A REMS document is a part of a REMS required by FDA and establishes the goals and requirements of the REMS.