
Membrane chromatography ensures purity at high flow rates.
Martha Pupo is a specialist in analytical development at the Centre for Genetic Engineering and Biotechnology

Membrane chromatography ensures purity at high flow rates.
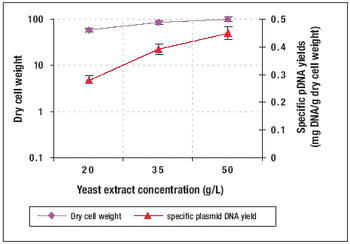
How to produce Plasmid DNA in a high-cell-density culture.
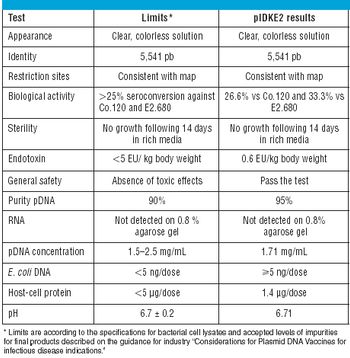

Published: February 1st 2010 | Updated:
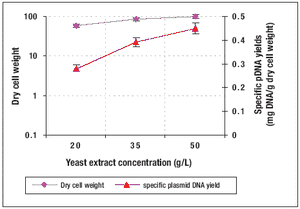
Published: July 1st 2009 | Updated:
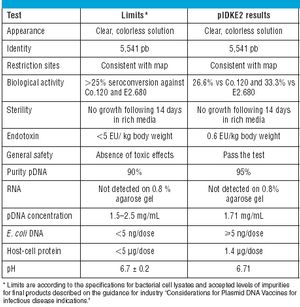
Published: September 1st 2008 | Updated: