
As the therapeutic landscape grows more complex, so too must the analytical techniques for cleaning validation to ensure the utmost cleanliness is achieved.

As the therapeutic landscape grows more complex, so too must the analytical techniques for cleaning validation to ensure the utmost cleanliness is achieved.

Effective cleaning and disinfection along with contamination controls are imperative when operating and utilizing a cleanroom.
Scientists can work to overcome the challenges associated with protein characterization through empowering technologies.

The need for improved analytics grows.
Establishing a data management strategy in-lab is a first step in generating successful analytical studies.
Establishing bioassay studies for biosimilar development is important for supporting regulatory filings.
Establishing analytical workflows for complex biopharmaceutical molecules can help predict their risk of degradation.

Developing analytical methods and bioassays is necessary from early on in the drug development life cycle.
Sophisticated analytical technologies are complementing traditional approaches in the identification of residual process-related impurities of biotherapeutics.

New challenges in extractable and leachable studies for cell and gene therapy products.

Assessing biosafety using NGS-based tests requires a continuum of skills in molecular biology, biocomputing, virology, and quality systems.

Bioanalytical studies are an important aspect of biologic drug development that may necessitate partnering with bioanalysis experts.

Characterizing and controlling protein aggregation is vital to ensure safety and efficacy of a biopharmaceutical product. In this interview, important aspects of protein aggregation and the tools available to address this issue are discussed.

As regulatory bodies extend the oversight of E&L testing, companies working with drug products need to make provisions on how to best comply with the evolving expectations.

Developing an effective bioassay is crucial for determining the potency of a drug substance or finished drug product. This article gives an overview of how to avoid most problems associated with correct bioassay development.

The following details some of the latest tools and systems available for biopharmaceutical laboratory operations.

Particulates or aggregates are a notable challenge for injectables, but there are several methods available to help with identification during formulation and development.

Stability testing for biologics is more complex than for small-molecule drugs, so companies should be aware of the potentially serious issues that can be costly and jeopardize drug development.

As regulatory guidance has evolved, changes in CCIT testing have also become apparent. In this article, possible CCIT strategy approaches are outlined.

Providing analytical data on the comparison between a biosimilar and the reference product is a primary consideration in the development of biosimilars.
Experts discuss best practices for performing glycan analysis.

The testing of raw materials is essential as raw material quality determines the outcome of biologic product quality.

This article discusses why it is important to apply risk analysis, QbD, and DoE in the development of analytical methods.

Materials in contact with a drug must be fully characterized to ensure they do not negatively affect the safety and efficacy of the product.
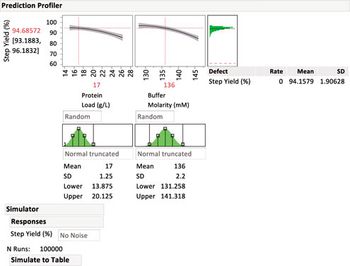
Statistical methods to identify critical process parameters and critical material attributes-and approaches to control them-are needed to protect drug product and drug substances.