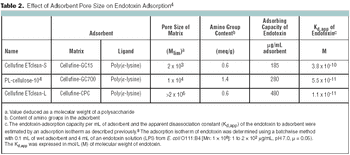
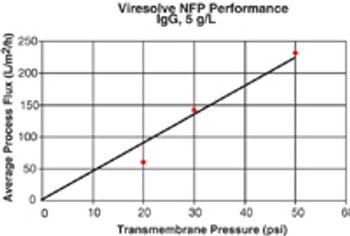
When you don't know the answer to a question, ask an expert. If the question is really big, ask more experts. If you have a collection of difficult questions, run a poll of many experts. That, in effect, was the impetus for Eden Biodesign to survey 670 BioPharm International subscribers with questions as to what will be the development mechanism to achieve safe, effective, and cheap new medicines.