
Researchers in China report test results for a microneedle patch for COVID-19 vaccine delivery.

Researchers in China report test results for a microneedle patch for COVID-19 vaccine delivery.

Lilly’s insulin, lyumjev, receives FDA approval for an expanded label.

ImmunityBio will proceed with a Phase I/II/III trial in South Africa of their dual-antigen T-cell vaccine as a boost in participants who were previously vaccinated against COVID-19.

Lonza further expands its micronization services with the acquisition of Swiss contract manufacturer, Micro-Macinazione, following its previous $5.5-billion acquisition of dosage form provider, Capsugel.

The initiative targets countries with less developed and evolving healthcare systems such as Latin America, South East Asia, and Africa.

Amgen discussed the drug-delivery approaches to two of its biologics in a recent second-quarter earnings call.

The wearable devices for the delivery of biologic products are now being manufactured and will be tested in clinical trials in the near future, according to the company.

US generics manufacturer Amneal acquires a former J&J plant in Ireland.

Industry experts spoke to BioPharm International about the key considerations in the development of a drug-delivery device for a biologic drug, the importance of human factors engineering, the advantages of prefilled syringes, and the challenges in the manufacture of these devices.

Gene and cell therapies represent the next-generation treatments for a wide range of diseases, but one challenge in the development of these therapeutics is the controlled delivery to the targeted site to maximize expression or engraftment while limiting systemic exposure.

Will AbbVie stave off biosimilar competitors by following Teva’s model for Copaxone?

Experts attending the European Psychiatry Association Congress in Vienna say that Adasuve has made an impact in the treatment of agitation in patients suffering from schizophrenia or bipolar I disorder.
Second-generation needle-free injection systems will make parenteral drug administration more convenient, efficient, and safe.

Drug spending rose last year at the highest rate since 2003, driven by specialty medicines, according to a report from pharmacy benefit manager Express Scripts.

The 2016 White House Budget proposes a change to the data exclusivity period for biologics and the authority to influence drug pricing.
Industry experts discuss the benefits and challenges of self-administration of injectable therapies.
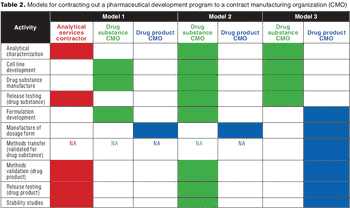
Formulation strategy is an important consideration when selecting and managing outsourced biopharmaceutical development programs.

The year 2007 witnessed the approval of fifteen biopharmaceuticals in the United States and European Union.

US Food and Drug Administration's Division of Biologic Oncology Products has approved two new biologics license application (BLA) supplements expanding the approval of Genentech's Herceptin (trastuzumab) for the treatment of breast cancer.