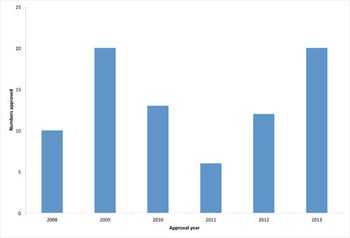
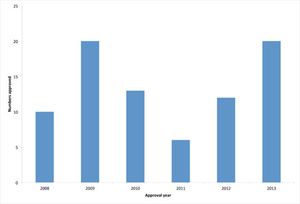
A review of the past year's trends in biopharmaceutical approvals shows an above average approval rate.
Associate Professor in the Industrial Biochemistry Program at the University of Limerick. He is also a member of BioPharm International's Editorial Advisory Board.

A review of the past year's trends in biopharmaceutical approvals shows an above average approval rate.
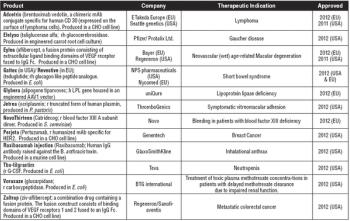
First gene therapy and plant-based expression vector products approved in 2012.
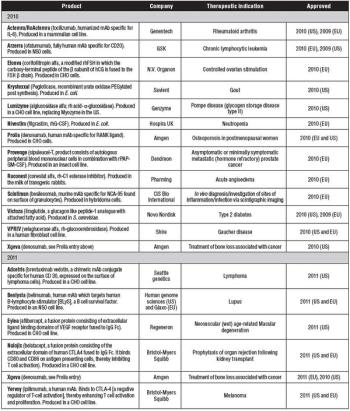
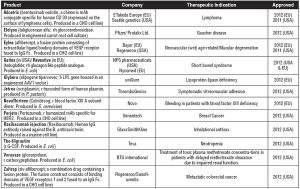
A review of new biologic drug approvals over the years, featuring highlights from 2010 and 2011.
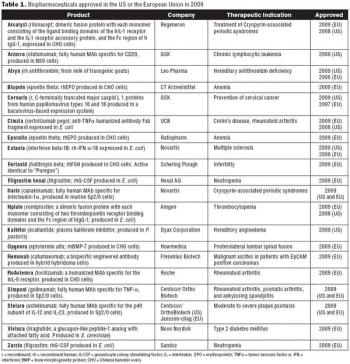
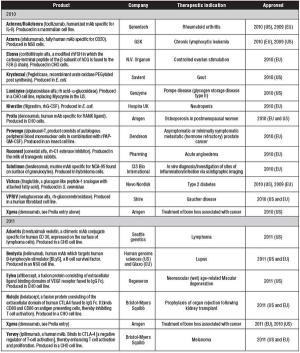
Biotech approvals were up last year. Is it a sign of a new trend?

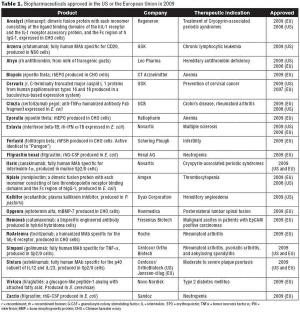
Ten biopharmaceuticals gained marketing authorization in the US or EU in 2008, although only four were new approvals.

The year 2007 witnessed the approval of fifteen biopharmaceuticals in the United States and European Union.

The book is a useful, comprehensive, and truly an excellent reference source of biopharmaceutical information.
An overall increasing proportion of future product approvals will be engineered in some way, either directly or indirectly.

There were notable approvals in nonparenteral delivery systems and biosimiliars in 2006.

In 2005, 10 biopharma- ceuticals gained marketing approval in the US or Europe, although only five of them were genuinely new molecular entities.
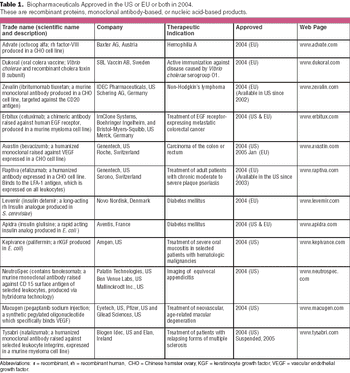
Cancer remains the primary indication (three products), again mirroring recent approval trends.
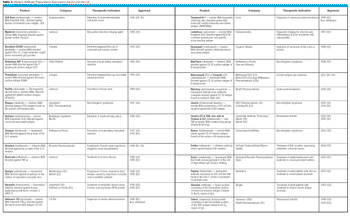
As of late 2004, 26 modern antibody-based therapeutic agents have been approved in the European Union and the US. Some 500 such products are currently in development, ensuring that the number of approved antibody-based products will increase substantially over the coming years.
Published: April 1st 2014 | Updated:
Published: April 1st 2013 | Updated:
Published: June 1st 2012 | Updated:
Published: October 1st 2010 | Updated:

Published: October 1st 2009 | Updated:

Published: April 1st 2008 | Updated: