
While a variety of innovations have already impacted the drug delivery landscape, improving sustainability and having the ability to make smaller volumes of drug products still require work.

While a variety of innovations have already impacted the drug delivery landscape, improving sustainability and having the ability to make smaller volumes of drug products still require work.

AstraZeneca reports that its combination antibody therapy, AZD7442, reduced chances of severe COVID-19 or death by as much as 67%.

The EMA’s Committee for Medicinal Products for Human Use has recommended marketing authorization approval for AstraZeneca’s benralizumab, a monoclonal antibody for treating severe asthma.

Nemera is now authorized to handle, assemble, sterilize, and store pharmaceutical drugs and medicinal products for autoinjector combination products at its facility in Neuenburg, Germany.
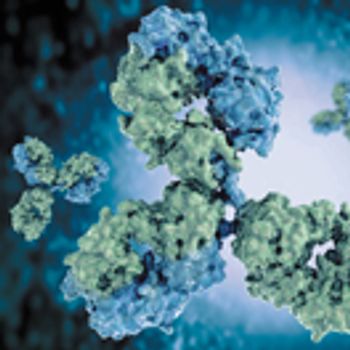
OrlaSURF technology can be used for the development of target-binding assays to monitor the binding of an ADC to its antigen.

Communication and taking the time to develop the process are key to successful transfer and scale up of biologics

Interactions between biologic drug products and the components of prefilled syringes can cause protein aggregation, but there are alternative materials that can help mitigate this problem.

The collaboration will provide GMP manufacturing ahead of future clinical studies.
Scale-up of complex, innovative products requires commercialization models that are sustainable.

Selecting a delivery method early on may be beneficial.

Surface plasmon resonance is helping define bispecific antibodies, the next-generation of biopharma therapeutics.

Although the Committee for Medicinal Products for Human Use (CHMP) gave Blincyto a positive opinion, full approval of the drug in the EMA will rely on additional clinical studies.

Under terms of the agreement, Amgen will license Xencor’s XmAb technology platform for five Amgen programs and one Xencor program.

Abenza acquired biopharmaceutical CDMO PacificGMP and expanded the company’s San Diego facility.

The collaborative effort will be focused on fully humanized antibodies.

Merus announced new investors and the sale of $80.5 million in shares to advance its immuno-oncology programs.

Industry experts spoke to BioPharm International about the key considerations in the development of a drug-delivery device for a biologic drug, the importance of human factors engineering, the advantages of prefilled syringes, and the challenges in the manufacture of these devices.

Novasep's new antibody drug conjugate facility at its site in Le Mans, France will be commissioned in 2016.
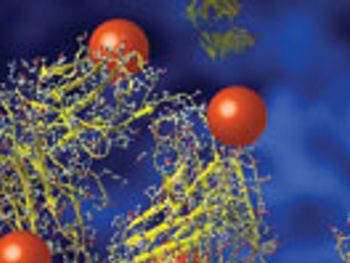
As ADCs move through the drug-development process, different analytical methods are often required.

Under terms of the agreement, Zymeworks could earn up to $164 million per successful drug candidate.

Roche will use Dutalys’ DutaMab technology for the engineering of bispecific therapeutic antibodies.Roche Acquires Bispecific Antibody Developer Dutalys

Genmab enters collaboration with Eli Lilly to use and evaluate Genmab's DuoBody technology for bispecific antibodies.

Clinical-stage biopharmaceutical company Ambrx leverages its site-specific bioconjugation technology through partnerships.

The first part of CPhI's Annual Expert Industry Report examines ADCs, single-use technology, and regulatory failure.