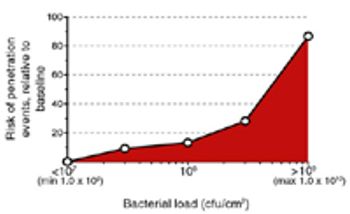
Filterability and bacterial retention must be verified very early in process development to ensure successful sterilizing filtration validation.

Filterability and bacterial retention must be verified very early in process development to ensure successful sterilizing filtration validation.

Adjuvant activity can be greatly improved by appropriate formulation of cytosine-phosphorothioate-guanine oligodeoxynucleotides (CpG ODNs).

Can vendor-user partnerships overcome the typical wait-and-see attitude toward new technologies?
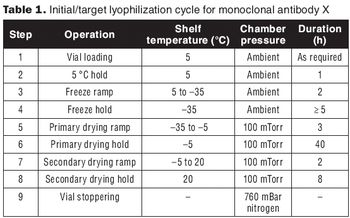
Develop a relevant design space without full factorial DoE.
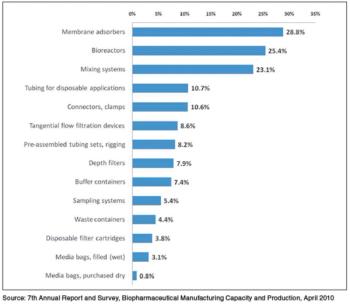
Although single-use systems are widely used in upstream unit operations, their acceptance in downstream processes has been slow.
An analysis of flow rate, load density, viral clearance, and cost.
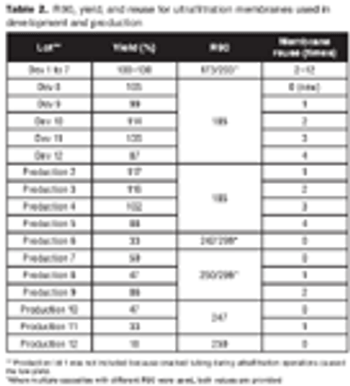
How to balance porosity, plugging, and lot-to-lot variability in filters.

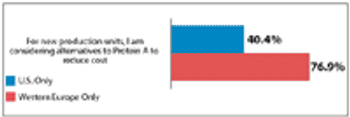
Biomanufacturers and vendors are now exploring more cost-effective purification technologies.

We all know that experience is the best teacher. With H1N1, we have a great opportunity to learn important lessons.
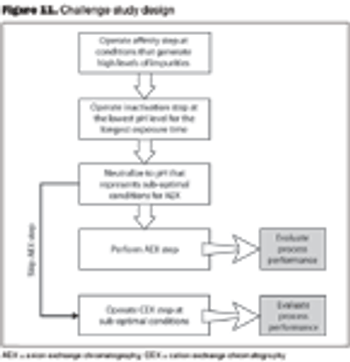
How to use risk assessment strategies to integrate operations.
A systematic approach facilitates formulation component selection.
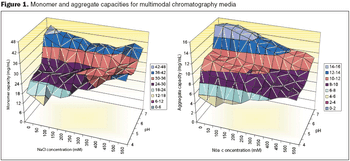
HTPD allows rapid screening of chromatographic parameters.

Characterizing the higher order structure (HOS) of protein drugs increases manufacturers' understanding of stability and batch-to-batch variability, and may make it possible to link variants or aggregates to safety and efficacy. Yet at the January 24 CMC Strategy Forum in Washington, DC, regulators expressed concern that methods to characterize the three-dimensional structure of proteins are not routinely applied to biotechnology products.
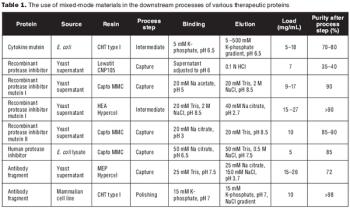
Salt-tolerant adsorption and unique selectivity are the major advantages of mixed-mode materials over single-mode resins.
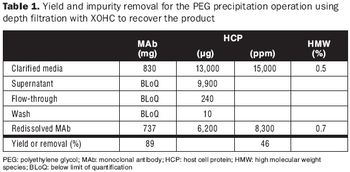
This alternative purification method to chromatography is readily scalable and fits a fully disposable downstream process.
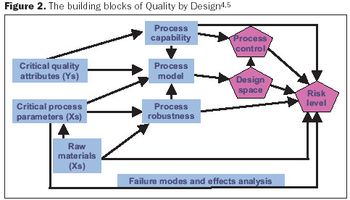
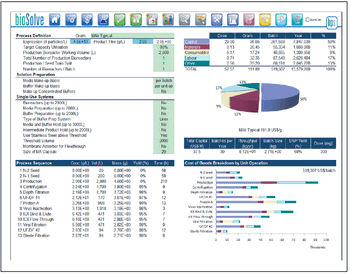
To speed up the downstream process, you must get the right data, in the right amount, at the right time. Here's how.
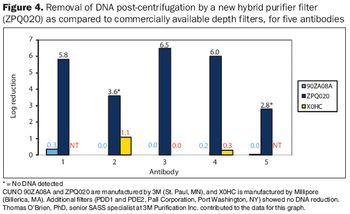
New technologies and adaptations of existing technologies can improve platform processes.
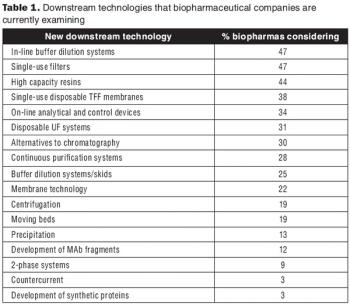
The new technologies being developed to improve downstream systems go beyond traditional chromatography.
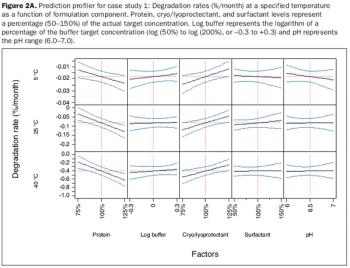
Design of experiments is a valuable tool for identifying aspects of a formulation that are critical to product quality.

Membrane chromatography ensures purity at high flow rates.
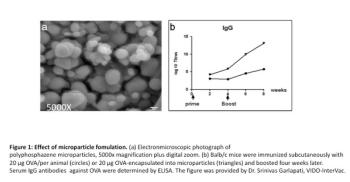
This article presents ongoing research at VIDO-InterVac for the development of safer and more effective adjuvants. These include adjuvants that stimulate innate immunity in the body and combination adjuvants.
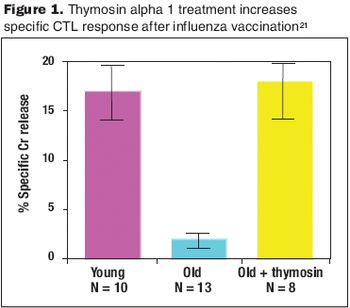
Clinical studies have shown that treatment with thymosin alpha 1 increases response to vaccination.
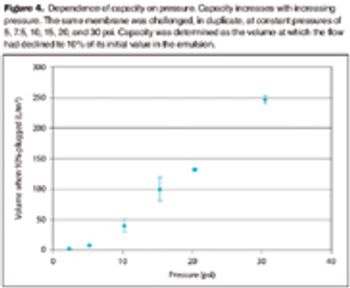
The viscosity of oily emulsions can reduce filter capacity and bacterial retention.