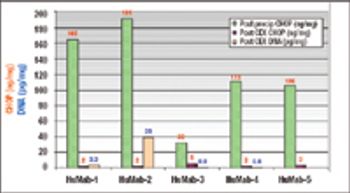
Precipitation prior to capture chromatography offers a simple, robust, and economical method to remove CHO host cell proteins and DNA.

Precipitation prior to capture chromatography offers a simple, robust, and economical method to remove CHO host cell proteins and DNA.
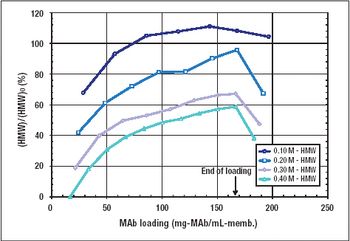
This alternative to column chromatography is suitable for flow-through as well as bind-and-elute purification operations.

New techniques can overcome bottlenecks in existing facilities.
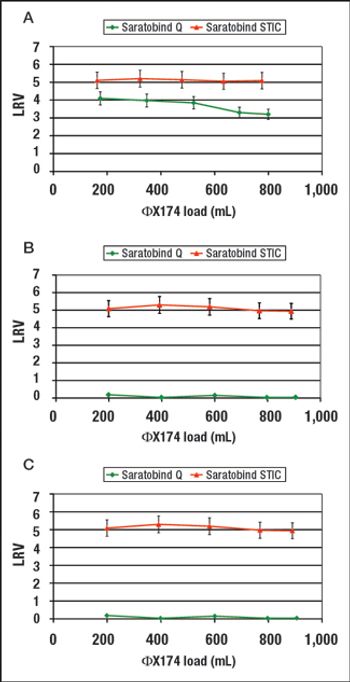
STIC allows polishing to be carried out without an interstitial dilution step, which reduces process time and avoids additional buffer preparation and hold steps.
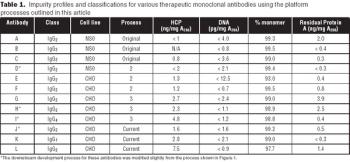
A purification scheme to maximize the efficiency of the purification process and product purity while minimizing the development time for early-phase therapeutic antibodies.

How to implement a risk-based approach to eliminate viruses using orthogonal technologies.

The industry and government must collaborate to develop robust technologies and quicker, more flexible manufacturing approaches for vaccine development.
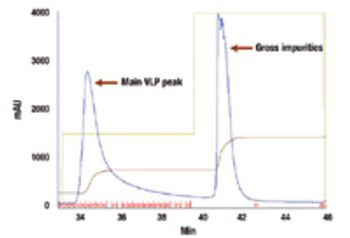
New technologies such as virus-like particles are promising weapons in the battle against pandemic influenza.

Ten biopharmaceuticals gained marketing authorization in the US or EU in 2008, although only four were new approvals.

Membrane-based TFF technology can ease scale-up and provide a higher recovery percentage compared to conventional purification methods.
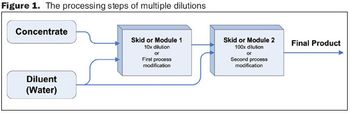
Automated in-line dilution can help solve capacity, financial, and quality concerns that biopharmaceutical manufacturing plants may be facing.

It is now possible to combine antigens with specific adjuvant systems to create more effective vaccines.
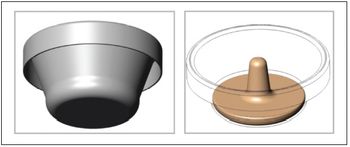
Needle-free vaccine delivery platforms can solve the problems of stockpiling, cold-chain management, and pandemic preparedness.

To assess current trends in separation and purification, BioPharm International turned to Mandar Dixit, head of product management?filtration technologies, Sartorius Stedim Biotech; Günter Jagschies, senior director of strategic customer relations, life sciences, biotechnologies, GE Healthcare; Richard Pearce, program director of purification, Millipore Corporation; and Jon Petrone, global technical director, Pall Life Sciences.
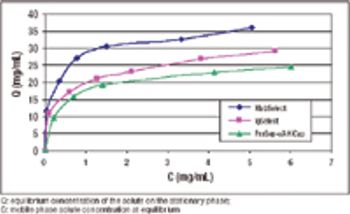
A stable alternative to Protein A chromatography.
Why staining is crucial in flow decay studies.
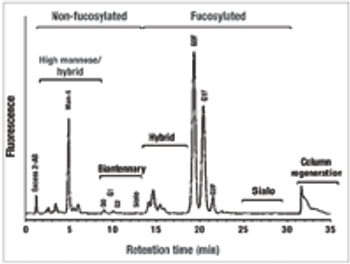
Select the best approach to determine critical quality attributes.
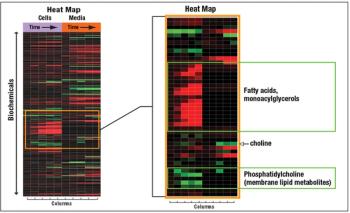
Through metabolomics, the metabolic underpinnings of cellular changes can be rapidly pinpointed, directing process development scientists to key areas for cell culture optimization.
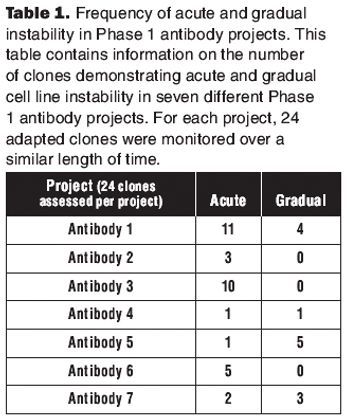
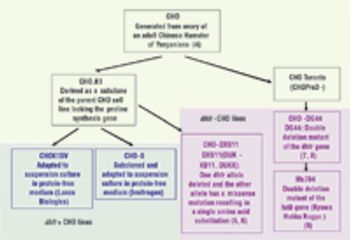
By considering stability as part of the cell line selection and cell banking paradigm, we can ensure that instability problems are not observed during clinical or commercial manufacturing.
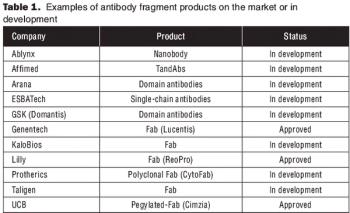
Microbial systems such as E. Coli and yeasts are most effective for producing antibody fragments.
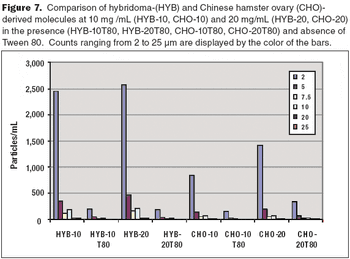
How to maintain product stability and prevent particulates.

A prove-free system monitors accurately at very small scale.

Biodefense start-up companies have an abundance of options when seeking funding.
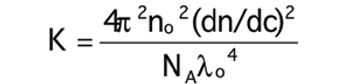
Can increase in ionic strength result in higher viscosity?