The authors present a robust and easy-to-implement chromatography column performance assessment method, called direct transition analysis (DTA).

The authors present a robust and easy-to-implement chromatography column performance assessment method, called direct transition analysis (DTA).

Increased understanding of potential impurities has spurred efforts to standardize monitoring procedures.

Extraction studies demonstrate approaches for evaluating single-use bio-pharmaceutical manufacturing materials.

Dedicated facility will address enhanced regulation of metals and impurities in pharmaceuticals.

Industry experts spoke to BioPharm International about the key considerations in the development of a drug-delivery device for a biologic drug, the importance of human factors engineering, the advantages of prefilled syringes, and the challenges in the manufacture of these devices.

The author discusses the various ways in which a quality-by-design program can enhance the extractable and leachable assessment of a drug product.
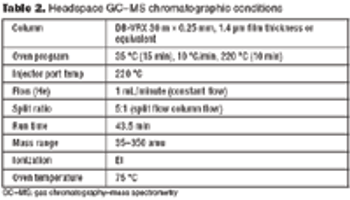
A systematic approach facilitates formulation component selection.

Two case studies illustrate a systematic approach.