
Enhanced sensitivity and ultra-high detection speed provide improved robustness and operability.

Enhanced sensitivity and ultra-high detection speed provide improved robustness and operability.

Analytical solutions are improving for raw material testing, process development, drug product release, and more.

The following details some of the latest tools and systems available for biopharmaceutical laboratory operations.
Effective application of mass-spectrometry tools can optimize biosimilar analysis, reducing development time and cost.
Real-time monitoring of product- and process-related impurities remains a challenge.
Drug developers must understand the complex bioanalytical assays for cell- and gene-therapy drug development programs and ensure that partners have the specialized expertise needed for complex therapeutic classes.

The companies will join forces on a research project to develop end-to-end workflows for the preparation, characterization, and monitoring of biotherapeutics using liquid chromatography-mass spectrometry.

Waters debuted a range of new TA Instruments innovations and the BioAccord System at Pittcon 2019 in Philadelphia, PA, on March 17–21, 2019.

Leveraging vast quantities of analytical data requires digitalization and platform integration.

The company has built a fit-for-purpose liquid chromatography–mass spectrometry (LC–MS) system to streamline analytical monitoring tests for biopharmaceuticals.
Microbial identity data can be critical for determining contamination sources.
By adapting techniques from other sciences-and exploring better tools for biologics drug development-researchers are addressing challenges of protein characterization.

Flush solutions for liquid chromatography mass spectrometry (LC-MS) systems and ultrapure solvents for ultra-high-performance LC-MS systems from Thermo Fisher Scientific can minimize interference and maximize lab instrument uptime.

The TSQ Fortis Triple Quadrupole Mass Spectrometer from Thermo Fisher Scientific offers fast, robust liquid chromatography-mass spectrometry (LC-MS/MS) analysis for clinical research laboratories.

More complex biologic samples must be evaluated to ever higher levels of specificity and sensitivity.

Materials in contact with a drug must be fully characterized to ensure they do not negatively affect the safety and efficacy of the product.

Process analytical technology tools have enabled manufacturers to monitor and control their production processes.

This article discusses affinity capture for tier determination and surveys a broad collection of commonly and less commonly used assays to analyze cell culture media

Access to multiple analytical techniques is essential for fully characterizing complex protein formulations.

Experts share insights on the various methods used for purity and impurity analysis of therapeutic proteins.

The critical quality attributes of biotherapeutics must be monitored to ensure product safety and efficacy.
The authors present a robust and easy-to-implement chromatography column performance assessment method, called direct transition analysis (DTA).

The author outlines an analytical strategy for establishing similarity in biosimilar development and approval.
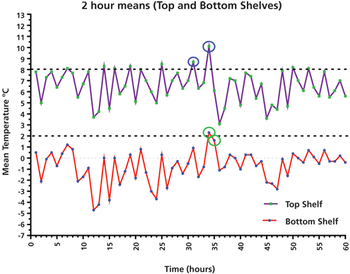
This column presents a data case study of a laboratory refrigerator and its qualification performance over five days, with important lessons for using average and individual results, as well as user requirements.
This article summarizes the approaches, challenges, and future perspectives for the characterization of N-glycans in biopharmaceutical products.