
The implementation of Industry 4.0 is projected to save time and cost for designing an optimal manufacturing process.

Anurag S. Rathore is a professor in the Department of Chemical Engineering at the Indian Institute of Technology Delhi and a member of BioPharm International's Editorial Advisory Board, Tel. +91.9650770650, asrathore@biotechcmz.com.

The implementation of Industry 4.0 is projected to save time and cost for designing an optimal manufacturing process.

Therapeutic protein aggregates can be characterized using image analysis algorithms coupled with microscopy techniques.

Image analysis algorithms coupled with microscopy techniques can be used to characterize aggregates of therapeutic proteins.

Image analysis algorithms coupled with microscopy techniques can be used to characterize aggregates of therapeutic proteins.
A novel mathematical approach for fitting concentration-response curves is proposed that offers a more accurate estimation of potency data.

This article summarizes industry views on PAT in bioprocess-related applications and presents a vision for the biopharmaceutical industry to achieve Industry 4.0.

The authors review some of the monoclonal antibody candidates that reached Phase III clinical trials but were discontinued at later stages.

The authors review some of the monoclonal antibody candidates that reached Phase III clinical trials but were discontinued at later stages.
The biopharmaceutical landscape of India is transforming in terms of regulatory policies, product development, and affordability.

The need for real-time monitoring and control has spurred the development of new analytical tools.
Amid the rush for a SARS-CoV-2 vaccine to deal with the COVID-19 pandemic, a robust risk assessment must be conducted, and mitigation strategies applied.
Analytical and functional characterization of virus-like particles enables process reproducibility and product consistency.

Advances in preclinical development play a crucial role in reducing cost for developing biosimilars.

This article presents some key differences between the US and European regulation of biosimilars, including naming conventions and pharmacovigilance of biosimilars, and the impact of biosimilars on commercialization and affordability of biotherapeutics.

A major advantage of SPR-based analysis is its ability to estimate the association and dissociation rate constants, an advancement over the traditional steady-state analysis of biomolecules.
Kinetic models can be used to study aggregation and fragmentation to help ensure stability.
Transcriptomics plays a role in influencing the production of recombinant therapeutics in microbial and mammalian hosts.
The approaches for sample preparation of preclinical evaluation of safety and efficacy are addressed taking into consideration the shortcoming with the contemporary approaches.

The authors review how media components modulate the quality of monoclonal antibody products

Chromatography modeling can enhance bioprocessing efficiencies.

A novel coiled flow inversion reactor (CFIR) improves process productivity and performance.

The authors review the technologies that may help bioprocessing become a truly continuous operation and present case studies that could contribute to the integration of upstream and downstream platforms.
The authors review the status of expression of antibodies in microbial hosts and present the recent advances in the production of aglycosylated antibodies in bacteria.
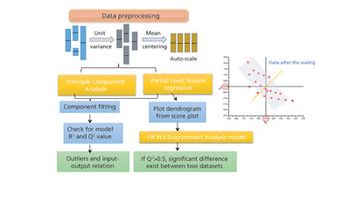
The authors review major developments in use of MVDA in bioprocessing applications.

Large-scale implementation of Protein A chromatography offers several challenges.
The authors take a look at the past and future impact of the Indian Pharmacopoeia Commission.
Establishing the CQAs of a mAb product by evaluating impact and uncertainty during risk assessment.
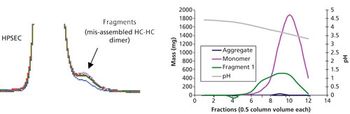
The authors discuss the evolution of the purification platform for manufacturing of mAb therapeutics.

The authors discuss the application of risk management in process lifecycle validation, manufacturing, and change control.

Aggregate formation is influenced by multiple aspects of the bioproduction process but can be mitigated by good process design and control.
Published: December 2nd 2020 | Updated:

Published: December 13th 2021 | Updated:
Published: November 1st 2021 | Updated:

Published: November 1st 2013 | Updated:

Published: August 1st 2013 | Updated:

Published: March 1st 2013 | Updated: