
Alternative approaches to freeze drying are gaining popularity and will help to overcome some of the challenges associated with conventional lyophilization.

Alternative approaches to freeze drying are gaining popularity and will help to overcome some of the challenges associated with conventional lyophilization.

By eliminating the waste involved in traditional approaches to project set-up and execution, integrated project delivery helps innovators in the European life sciences market meet their fast-track delivery targets.

Better understanding and control of cell behavior is yielding benefits, upstream and beyond.
Accelerated approval pathways and growing demand for cell and gene therapies are putting pressure on providers of cellular starting materials, and they must ensure a steady supply.
Connectors are a critical element in the process optimization of single-use bioprocessing systems.

The use of external retainers to enhance the seal between connectors and tubing is an essential component in single-use manufacturing systems.

A streamlined approach may enhance process efficiency and product quality.

An understanding of continuous process validation can lead the way to consistent approaches, reduced investigation times and observations, the avoidance of lost batches, and high-quality products.

Integrating advances in facility design can meet differing and emerging bioprocessing needs.

Making the switch from batch to continuous manufacturing requires a thorough understanding of the process.

Switching grades of raw material late in the development cycle can be costly. Best practice says get it right at the beginning.

Unexpected problems can become opportunities to share knowledge and improve processes.
Defining best biopharmaceutical practices is necessary to ensure the safety of the supply chain.

The biopharmaceutical industry is developing a new approach to controlling variability in raw materials.

For single-use systems, supply chain excellence requires a commitment to problem solving across organization boundaries.

Using closed systems opens up many new possibilities for how facilities are designed and operated and may also present lower risk to the operation and, ultimately, the product.

Maintaining flexibility in biopharmaceutical manufacturing can deliver positive results.

Benchmarking can be a useful tool to improve manufacturing practices.

Improvement strategy should be linked to business strategy.

Is process-centered organization in biopharmaceutical manufacturing a stepping stone or a stumbling block?

A one-day sign off for batch records is considered a best practice in the industry.
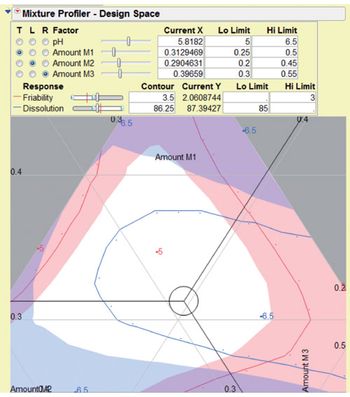
Harmonized regulations call for a risk-based and systematic approach to evaluating and selecting CPPs.