
The need for real-time monitoring and control has spurred the development of new analytical tools.

The need for real-time monitoring and control has spurred the development of new analytical tools.

Analytical solutions are improving for raw material testing, process development, drug product release, and more.

The following details some of the latest tools and systems available for biopharmaceutical laboratory operations.

Waters debuted a range of new TA Instruments innovations and the BioAccord System at Pittcon 2019 in Philadelphia, PA, on March 17–21, 2019.

Leveraging vast quantities of analytical data requires digitalization and platform integration.
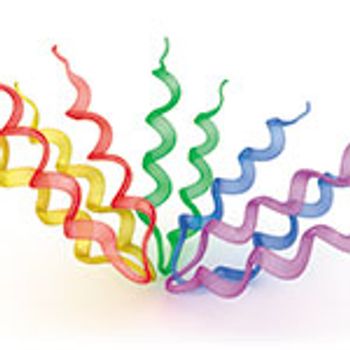
By adapting techniques from other sciences-and exploring better tools for biologics drug development-researchers are addressing challenges of protein characterization.

More complex biologic samples must be evaluated to ever higher levels of specificity and sensitivity.

Process analytical technology tools have enabled manufacturers to monitor and control their production processes.
The National Institute of Standards and Technology (NIST) has developed Standard Reference Material (SRM) 2082 as a pathlength standard for UV absorbance measurements for use with the new generation of microvolume spectrophotometers and short-pathlength cuvettes.

Spectroscopic tools present an alternative method for reliable at-line process monitoring and control.
The authors present a robust and easy-to-implement chromatography column performance assessment method, called direct transition analysis (DTA).
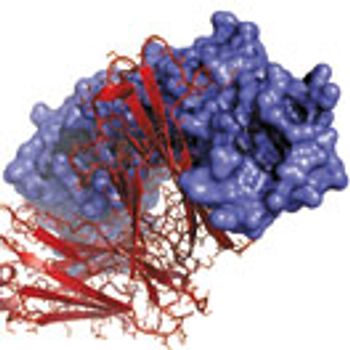
An increase in biologics raises awareness of particle generation and its role in negative patient outcomes.

Experts discuss recent advances in cell viability testing methods in bioreactors.
Data acquired from osmolality, glucose, and folic acid tests provides useful information for the specific identification of cell-culture media.

Researchers describe a new method to compare the higher-order structure of a reference biologic with its proposed biosimilar product candidates.
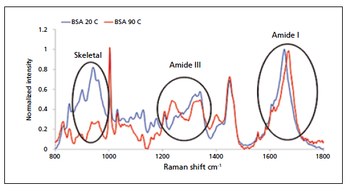
In this article, the author reviews some of the techniques that can yield valuable information on protein stability, focusing specifically on protein aggregation. Emphasis is placed on the enhanced information made available when technologies are used orthogonally, and the alignment of different approaches with specific stages of the biopharmaceutical development workflow.

The European Pharmacopoeia rewrites in its general chapter on Raman spectroscopy.
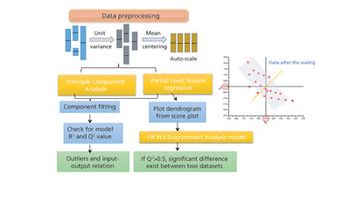
The authors review major developments in use of MVDA in bioprocessing applications.

This article presents first-hand perspectives from industry users to suppliers of single-use sensors.

MedImmune will provide funds and access to monoclonal antibodies to seven postdoctoral associates for the creation of protein measurement and characterization tools.
Understanding and preventing protein aggregation is crucial to ensuring product quality and patient safety.

MedicalRF.com/Science Photo Library-ANDRZEJ WOJCICKI/Henrik Jonsson/Stocktrek Images; Dan Ward
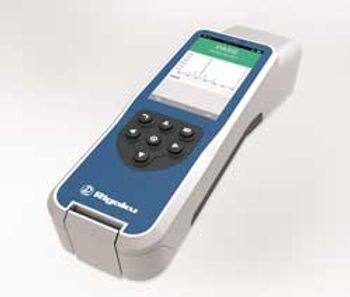
Handheld Analyzer Improves Accuracy

Rigaku Raman Technologies announced an updated version of the FirstGuard handheld Raman analyzer at Pittcon 2013.

Applying quality-by-design and process analytical technology facilitates process understanding and control of various operations in lyophilization.