Effective application of mass-spectrometry tools can optimize biosimilar analysis, reducing development time and cost.

Effective application of mass-spectrometry tools can optimize biosimilar analysis, reducing development time and cost.
Real-time monitoring of product- and process-related impurities remains a challenge.
Drug developers must understand the complex bioanalytical assays for cell- and gene-therapy drug development programs and ensure that partners have the specialized expertise needed for complex therapeutic classes.

Laboratory test methods evaluate cleaning agents and cleaning process design for removing resin residues from the surfaces of non-dedicated chromatography columns systems.

Leveraging vast quantities of analytical data requires digitalization and platform integration.

Determining a peptide’s purity is challenging because impurities are often structurally similar to each other and the API and can be present at very low concentrations. New approaches offer a solution.

The company has built a fit-for-purpose liquid chromatography–mass spectrometry (LC–MS) system to streamline analytical monitoring tests for biopharmaceuticals.
By adapting techniques from other sciences-and exploring better tools for biologics drug development-researchers are addressing challenges of protein characterization.
Analytical exoglycosidases are transitioning from being largely academic tools to being suitable for glycan analytics in biopharmaceutical manufacturing.

As it investigates the root cause of an impurity discovered in valsartan, FDA extends its studies to APIs with similar synthesis processes.

Flush solutions for liquid chromatography mass spectrometry (LC-MS) systems and ultrapure solvents for ultra-high-performance LC-MS systems from Thermo Fisher Scientific can minimize interference and maximize lab instrument uptime.

More complex biologic samples must be evaluated to ever higher levels of specificity and sensitivity.

The company’s next-generation ultraperformance liquid chromatography platform is designed to meet the evolving laboratory requirements for chromatographic performance.

Access to multiple analytical techniques is essential for fully characterizing complex protein formulations.

New, user-friendly analytical systems provide high-throughput comprehensive characterization and quantitation of small and large molecules.

Spectroscopic tools present an alternative method for reliable at-line process monitoring and control.

The critical quality attributes of biotherapeutics must be monitored to ensure product safety and efficacy.
The authors present a robust and easy-to-implement chromatography column performance assessment method, called direct transition analysis (DTA).

Process analytical testing for biopharmaceuticals requires enhanced methods due to complex bioprocesses.
A UHPLC SEC approach for protein aggregate analysis of mAbs is presented.

This study outlines methods for an alternative protein-polishing process for challenging proteins.

The author outlines an analytical strategy for establishing similarity in biosimilar development and approval.
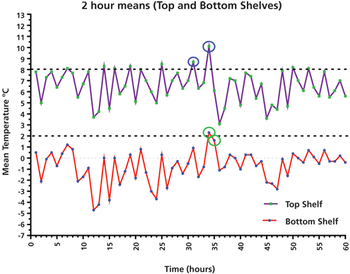
This column presents a data case study of a laboratory refrigerator and its qualification performance over five days, with important lessons for using average and individual results, as well as user requirements.
This article summarizes the approaches, challenges, and future perspectives for the characterization of N-glycans in biopharmaceutical products.

Increased understanding of potential impurities has spurred efforts to standardize monitoring procedures.