
Grace's ProVance disposable Protein A chromatography column is designed for downstream purification.

Grace's ProVance disposable Protein A chromatography column is designed for downstream purification.

OPUS technology designed to process large single-use bioreactor harvests.
The biopharmaceutical industry is developing a new approach to controlling variability in raw materials.

Research uncovers trends and factors affecting the pre-packed disposable chromatography columns market for downstream bioprocessing.

Experts discuss the advances in single-use technology for downstream bioprocessing applications.

Experts discuss the advances in single-use technology for downstream bioprocessing applications.

UPLC System for Nano- to Microscale Separations

Researchers are using current understanding of the lyophilization process to predict performance on many levels during both process development and manufacturing.

ALpHA G Capsule Filter for Single-Use Systems
Stain-Free Chromatography Workflow

A novel approach to sterile drug product manufacturing that uses a single-use assembly in a multi-product final filling suite with isolator technology offers benefits of efficiency and flexibility.
Advances in instrumentation, software, and methodologies provide more information than ever on the higher-order structure of proteins.

Automated sample handling, advanced glycan analysis and specially designed columns are helping biosimilar manufacturers speed up confirmation of the biosimilarity of their products.

Single-use systems and other technologies drive process efficiencies, but there is room for improvement.

With SMEs gaining wider recognition as the powerhouse of research and innovation in Europe, regulatory agencies are urging companies to engage with regulators early in the drug-development process.
Antibody fragments pose unique challenges in recovery, purification, and formulation.

Techniques to enable the design and formulation of stable, protein-based therapeutics.

A prototype Protein A resin is evaluated for purification performance, reusability, and cost performance.

Data from BioPlan Associates' 10th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production suggest that the interest in disposable devices has begun to extend to biopharma operations beyond basic single-use bags and connectors.
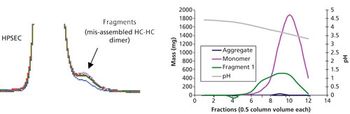
The authors discuss the evolution of the purification platform for manufacturing of mAb therapeutics.

BioReliance opens Clearance Services Laboratories in Maryland for downstream bioprocessing studies.

While the severity of capacity problems related to downstream processing appears to have eased, it continues to be a problem and chromatography columns are the most frequent culprits.
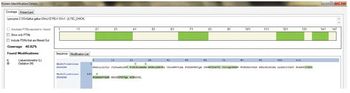
A simple, sensitive, and universal platform for identification and quantification of trace-level protein impurities in biotherapeutics is described.

Sartorius Stedim Biotech's Sartoguard NF prefilter series features a combination of high-performance PES and innovative nano-fleece technology.

Tosoh Bioscience's two ion exchange chromatography resins are designed for the high-resolution purification of proteins, peptides, and oligonucleotides.