Early Phase Development
Latest News
Latest Videos
More News

Treatment for cancer continues to drive immense innovation in the drug development pipeline, with advanced cell therapies, vaccines, and ADCs taking center stage.
Platform processes and effective risk assessments help overcome time and cost challenges.

The authors have reviewed applications of novel technologies in the major stages of biosimilars development: process development, pharmacology, toxicology, and clinical trials, with an emphasis on recent regulatory requirements.

With the Proteologix acquisition, Johnson & Johnson gains two bispecific antibody early phase assets for immune-mediated diseases.

Broken String Biosciences and the Francis Crick Institute will collaborate on research into how genome stability impacts ALS.

Under a global license and collaboration agreement, AbbVie and OSE Immunotherapeutics will aim to develop OSE-230, a mAb for treating chronic inflammation.

With the formation of the new R&D unit, Regeneron will assume full development and commercialization rights to 2seventy bio’s preclinical- and clinical-stage cell therapy pipeline.

The company is currently developing EVX-101 as an adjunctive treatment for MDD due to patients experiencing an inadequate response to first-line antidepressants such as selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors SNRIs.

Stable producer cell lines show real promise despite continued development challenges.

Chime Biologics, Leads Biolabs, and BeiGene have formed a three-way collaboration to advance the development and global manufacturing of Leads Biolabs’ lead mAb candidate, LBL-007.
Despite many development challenges, stable producer cell lines show real promise.

Lives are saved when time from vein to vein decreases.

Key challenges posed to autologous and allogeneic treatments could be resolved by in-vivo CAR-T gene therapies.
Decreasing vein to vein time saves lives.
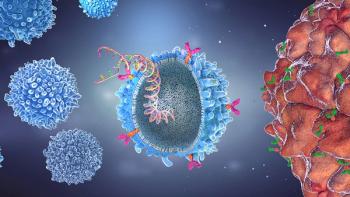
In vivo CAR-T gene therapies could overcome the challenges faced by autologous and allogeneic treatments.

An early drug candidate screening strategy should incorporate clear targets to lessen late-stage failure.

Preclinical testing is better able to evaluate complex drug candidates thanks to innovations in animal model approaches.

Precision BioSciences has released clinical trial data on CAR T therapy candidates PBCAR0191, PBCAR19B, and PBCAR269A.

To build trust and ensure a high-quality product, companies must be sure their contracts are firm and their lines of communication even firmer.

New technologies are treatments are emerging to tackle COVID-19 and its assortment of new variants.
Although mRNA and viral vector vaccines have been top of mind for COVID-19 treatments, other technologies and treatments are emerging—along with new variants.

Sanofi’s acquisition of Origimm Biotechnology will add to its pipeline of vaccine candidates for treating acne.

Secarna Pharmaceuticals and Achilles Therapeutics have entered into an agreement to optimize the development of T cell therapies in Achilles’ pipeline.

The biopharma industry is seeing more merit in strategizing clinical and commercial drug development as early as the preclinical phase.

MilliporeSigma, the U.S. and Canadian Life Science business of Merck KGaA, Darmstadt, Germany, signed an agreement with Cellecta to license its genome-editing tool.