
The authors describe the growth characteristics of human mesenchymal stem cells cultured in a stirred-tank bioreactor.

The authors describe the growth characteristics of human mesenchymal stem cells cultured in a stirred-tank bioreactor.

INTERPOL and 29 of the world's largest pharmaceutical companies have joined forces in an initiative to battle counterfeit drugs.

A Q&A with SAFC and BioReliance about sourcing and risk mitigation for raw materials.
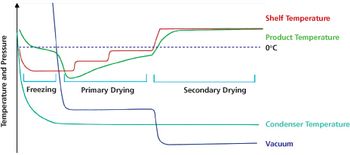
Optimized freeze-drying cycles can offer scientific and business advantages.

Aggregate formation is influenced by multiple aspects of the bioproduction process but can be mitigated by good process design and control.

BioPharm International spoke with industry experts about the effect FDA's 2011 process validation guidance has had on industry.
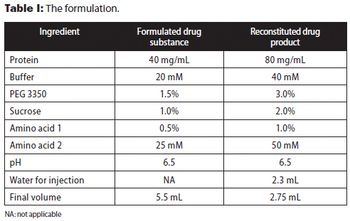
The authors present approaches used to reduce reconstitution time of a lyophilized high-concentration protein drug product.

Applying quality-by-design and process analytical technology facilitates process understanding and control of various operations in lyophilization.

Kevin Ott, executive director of the Bio-Process Systems Alliance, discusses the latest trends and issues surrounding single-use technologies and the role of his association.

Merck and Samsung Bioepis have formed an agreement to develop and commercialize multiple prespecified and undisclosed biosimilar candidates.

FDA has released a list of more than 50 guidance documents planned for 2013.

FDA has released a list of more than 50 guidance documents planned for 2013.

NIBRT's Michael Lacey provides an overview of biopharmaceutical facility design and operation.

A look at vaccine history, markets, manufacturing, and overcoming the scale-up dilemma.

Improvement strategy should be linked to business strategy.

Rutgers engineers constructed a direct compaction line in collaboration with Janssen.

Therapeutics targeting epigenetic mechanisms of disease will change the pharmaceutical marketplace.

What the industry's future holds and what needs to be done to get there.

NIBRT's Ray O'Connor provides an overview of aseptic processing.

Recent research examines the sequence of events underlying cellular reprogramming, which may aid in better control of the production of induced pluripotent stem cells.

This month, Sharon Strause, an industry consultant, provides a look back at "Computer System Validation Part I: Testing and Verification of Applications Software" by Leonard J. Goren.

The European Commission (EC) is seeking to introduce a black symbol to identify medicines that are subject to additional monitoring.

An operation spanning 16 African countries and conducted by the World Customs Organization (WCO) in partnership with the Institute of Research against Counterfeit Medicines (IRACM) led to the seizure of more than 82 million doses of counterfeit medicines.
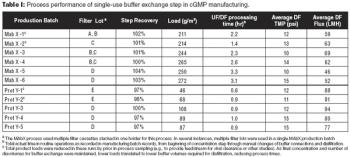
This case study describes the process used to transition from a multi-use system to single-use tangential flow filtration for performing final buffer exchange steps.

Industry experts from GE Healthcare, Tarpon Biosystems, Polybatics, Merck Millipore, and Repligen discuss the challenges of adapting disposable technology to the chromatography process.