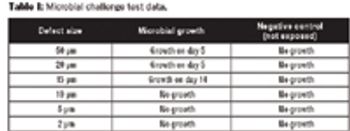
Defects as small as 10 μm can be detected without compromising product cleanliness using helium integrity testing.

Defects as small as 10 μm can be detected without compromising product cleanliness using helium integrity testing.

In this quarter's column, highlights from the IBC Single-use Applications meeting, the PDA Single-use Workshop, and the BioProcess systems Alliance International Single-Use Summit are presented.

Single-use systems continue to gain traction among biomanufacturers, especially CMOs.
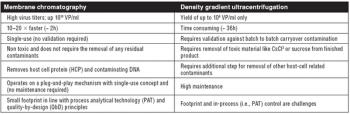
An evaluation of the technologies needed to develop a safe, effective, and economically efficient vaccine. This article is part of a special section on vaccines.

A Q&A with Rick Hancock, president of Althea Technologies. This article contains bonus online material.
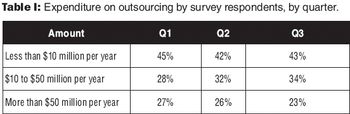
A survey provides insight into drug companies' plans for spending on outsourced services. This article contains bonus online material.
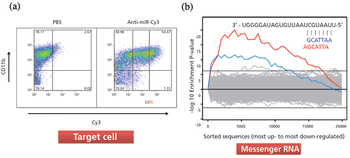
The authors provide insight into microRNA biology, and the simplicity of anti-miR oligonucleotide drug delivery.

The International Society for Pharmaceutical Engineering (ISPE) will soon publish an update for its guide to sterile-product manufacturing facilities. The new publication will replace the original guide, ISPE Baseline Guide: Sterile-Product Manufacturing Facilities, and contain practical information about technological advances in sterile manufacturing.

Developing a quality agreement template for single-use systems.
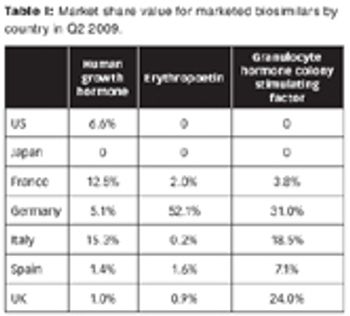
The market landscape for biosimilars is in flux, with limited penetration now, but with the potential for growth for those who can navigate the market. Plus: A SWOT analysis of biosimilars by Anjan Selz.

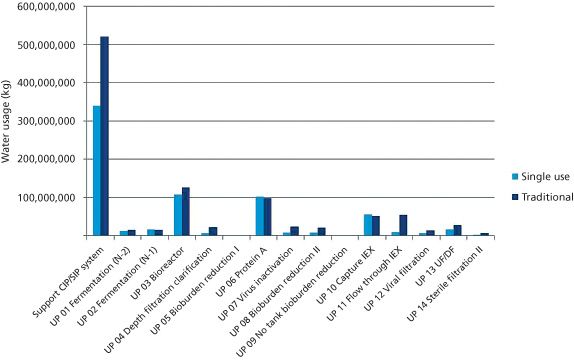
The author looks at strategies to minimize particle levels in the finished product when using single-use technologies downstream of final capabilities.
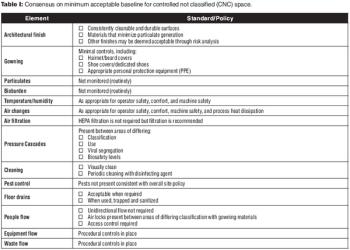
The authors re-examine environmental controls in the context of technical advances in manufacturing.

Incorporating regulatory requirements into the product life cycle is crucial.
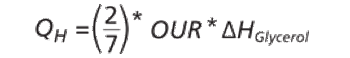
The authors describe challenges faced in transfer and scale-up of a fermentation process.

Plasmid DNA-encoding proteins offer many advantages, which are now being used in clinical trials.
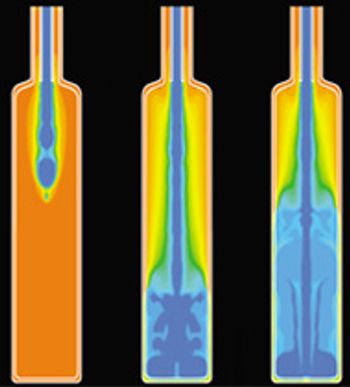
The authors give special consideration factors affecting blow–fill–seal technology.

Single-use conference roundup.
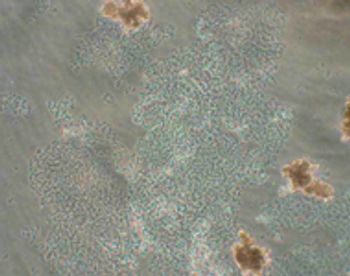
The authors present a case study identifying a contaminant.
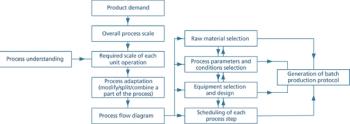
Clear documentation and open communication are essential for effective technology transfer.

Addressing supply-chain challenges.

Nigeria Looks to Simple Packaging Controls and International Cooperation to Curb Counterfeit Drugs

Supply-chain analytics can lead to increased profitability.

The authors present lessons learned from a case study of the transfer of a cell culture biotherapeutic process to a CMO.
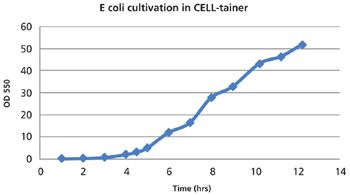
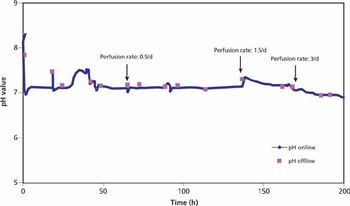
The authors discuss the use of single-use bioreactors.