
The YSI Sitini Online Sampler draws fluids from a bioreactor and delivers the samples to an analyzer.

The YSI Sitini Online Sampler draws fluids from a bioreactor and delivers the samples to an analyzer.

FDA issues a Form 483 to Wockhardt for quality issues at Morton Grove, IL facility.

Innovative polyethylene film sets new benchmark

PharmaCell has finalized its purchase of the cell therapy production facility from TiGenix.

The European Pharmacopoeia Commission re-evaluates its policy on the development of monographs for finished drug products.

Different approaches to prepare highly concentrated feed media for fed-batch Chinese hamster ovary cell culture are evaluated.
Suppliers see challenges to the adoption of single-use technologies for downstream processing as opportunities.

New formulations and expanded vaccine production are encouraged.

Progress is being made in the development of harmonized best practices for single-use systems.

New approaches to vaccine production are targeting rapid supply for pandemic situations and broadly effective therapeutic treatments.

Clinical Syringe Packages Increase Productivity

New human and plant-based expressions systems can enable faster product development and improve quality at potentially lower costs.

Integrated Single-Use Sterile and SIP Connector Provide Flexibility
Review regulatory requirements and the use of viral-challenge studies in drug development.

Aseptic Valve Range Increases Sterility

TAP Biosystems announced that Gallus BioPharmaceuticals is using its ambr15 micro bioreactor system to optimize process development and clone selection of novel antibody therapeutics and biosimilars.

Grace's ProVance disposable Protein A chromatography column is designed for downstream purification.

ThioBridge linker technology to attach different payloads to a range of antibodies

Biotechnology developer American CryoStem and Rutgers University partner on stem-cell platform technology development.

New identifiers and tracking requirements aim to block illegitimate products.
The biopharmaceutical industry is developing a new approach to controlling variability in raw materials.
The approval and acceptance of monoclonal antibody biosimilars is necessary if the biosimilars market is to experience real growth.
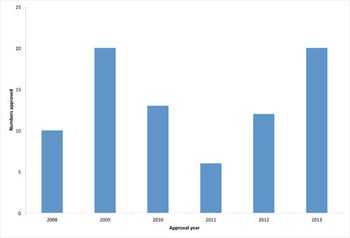
A review of the past year's trends in biopharmaceutical approvals shows an above average approval rate.

Vetter launches new serialization process to support track-and-trace efforts.

Displays will include new products for anticounterfeiting, labeling, and compliance packaging and new equipment for filling, aggregating, and quality control.