
Alvotech plans investment in biosimilars portfolio and manufacturing facility.

Alvotech plans investment in biosimilars portfolio and manufacturing facility.

TxCell has received manufacturing accreditation status of its cell-therapy production site in Besancon, France, from the French National Agency for Drug Safety (ANSM).

Drug Quality and Security Act gives FDA authority over compounding pharmacies.

A prototype Protein A resin is evaluated for purification performance, reusability, and cost performance.

New policies and products seek to maintain access to pain medicines while curbing rampant abuse.

Wolfgang Weikmann of Vetter Pharma discusses the implementation of quality by design in sterile manufacturing.
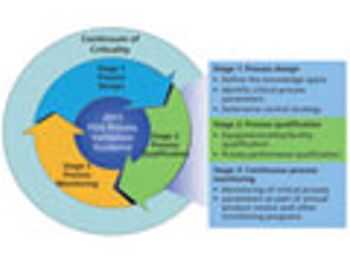
Criticality is used as a risk-based tool to drive control strategies.

The international “Fight the Fakes” campaign will raise awareness about the dangers of counterfeit medicines.

Almac releases an enhanced third-party logistics customer billing application.

GlaxoSmithKline plans to invest nearly $41 million to expand its operations at its facility in Montrose, the United Kingdom.

European Medicines Agency announces the launch of the Accelerated development of vaccine benefit-risk collaboration in Europe (ADVANCE) project.

Merck KGaA plans to build a new biomanufacturing facility in Nantong, China.

Phase I/II study to evaluate treatment of patients with melanoma using Pfizer’s palbociclib and GSK’s trametinib.

Covance Inc. and Pathoquest have announced a collaboration to provide next-generation sequencing based biosafety assessments to detect and identify viral contaminants within biologic compounds.

Ei Lilly announces investments in insulin manufacturing capacity for its sites in Indianapolis, Puerto Rico, France, and China.

Schott’s glass vials are specially manufactured to control and reduce delamination potential.

AstraZeneca will build a new facility in the United Kingdom to produce Zoladex, an injectable prostrate cancer treatment.

Lyra Myers, associate director and value creation agent at Roche, has been elected DCAT president.

Grifols expands and diversifies portfolio with acquisition of diagnostic business.

ISPE’s survey is industry’s first large-scale effort to collect data on patient experiences with clinical trial materials.

Using closed systems opens up many new possibilities for how facilities are designed and operated and may also present lower risk to the operation and, ultimately, the product.

BioPharm International speaks with industry experts about challenges faced in managing the cold chain.

Legislators agree on a limited bill affirming FDA authority over compounders while setting up a process for national drug tracking.

USP is developing and revising distribution standards in response to changes in the global supply chain.

Data from BioPlan Associates' 10th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production suggest that the interest in disposable devices has begun to extend to biopharma operations beyond basic single-use bags and connectors.