Insights on single-use systems implementation and exploitation in biopharmaceutical manufacturing and processing, based on a QbD approach.

Insights on single-use systems implementation and exploitation in biopharmaceutical manufacturing and processing, based on a QbD approach.

Is process-centered organization in biopharmaceutical manufacturing a stepping stone or a stumbling block?
Scaling up stem-cell cultures requires careful consideration of the bioreactor design.

Creating an effective nucleic acid-based vaccine requires protecting the fragile nucleic acid from degradation, effective transfection of the targeted cells, and producing high enough levels of antigen to evoke a robust immune response.

The author considers the types of tubing available to the industry and how to make an informed selection.

Patrick Jackson of Vindon Scientific offers key considerations for choosing an outsourced sample storage facility.

This article discusses the evaluation of a novel single-use fluidized bed centrifuge for harvesting of antibodies.

New US Pharmacopeial Convention (USP) standards provide a universal approach to organizing labels for prescription containers dispensed by US pharmacists in an effort to improve patient understanding.

Takeda's US subsidiary, Takeda America Holdings, is to acquire the vaccine specialist company LigoCyte Pharmaceuticals for an upfront payment of $60 million in a move intended to bolster and expand the company’s vaccine business.


Marco Chacon of Paragon Bioservices discusses the challenges associated with outsourced vaccine manufacturing.
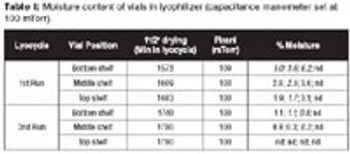
Using an alternate moisture-generation method may provide more accurate data for regulatory submissions.

Anne Marie Dixon of Cleanroom Management Associates, gives us an update on her 1988 article, "Clean Room Management."

ISPE has published a new guidance titled ISPE Good Practice Guide: Quality Laboratory Facilities, which defines design guidelines for quality laboratories.

Ties between the biotechnology industry and university research are crucial.

Using a competency-based approach to effectively train biopharmaceutical industry staff.
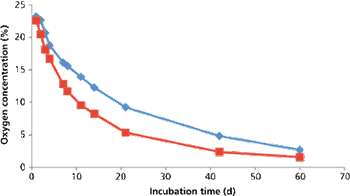
The authors describe a validation master plan for closed-vial filling technology.

Manufacturers and regulators struggle to control phony versions of crucial medicines.

There is no harmonized guidance on pre-use integrity testing of sterilizing filters, prompting discussion among users as to whether such testing is necessary.

A one-day sign off for batch records is considered a best practice in the industry.
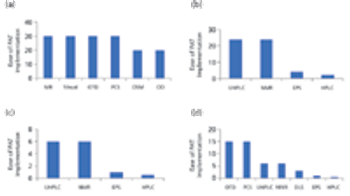
The authors review the various analytical methods that can enable use of PAT.

A report from the European Commission shows that fake pharmaceuticals were the top articles detained by European-Union customs in 2011.
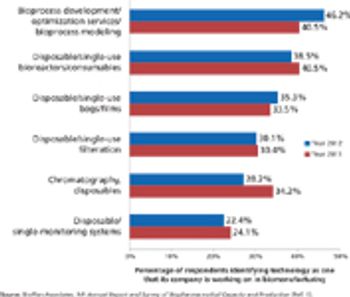
Industry wants more innovation, but can suppliers meet customers' needs?
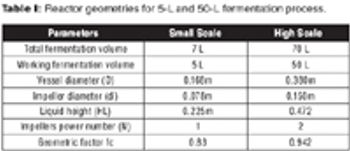
The authors present scale-up from a 5-L fermentor to a 50-L pilot-scale using the criterion of constant power consumption per unit liquid volume.
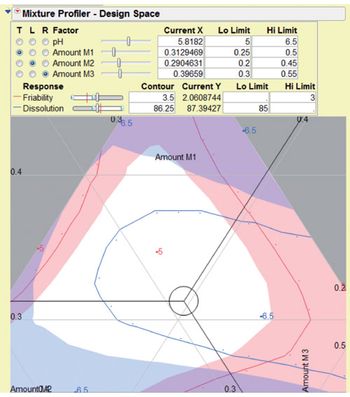
Harmonized regulations call for a risk-based and systematic approach to evaluating and selecting CPPs.