
Integrating advances in facility design can meet differing and emerging bioprocessing needs.
Simon Chalk is director of the BioPhorum Operations Group, simon@biophorum.com

Integrating advances in facility design can meet differing and emerging bioprocessing needs.

Switching grades of raw material late in the development cycle can be costly. Best practice says get it right at the beginning.

Unexpected problems can become opportunities to share knowledge and improve processes.
Defining best biopharmaceutical practices is necessary to ensure the safety of the supply chain.

The biopharmaceutical industry is developing a new approach to controlling variability in raw materials.

For single-use systems, supply chain excellence requires a commitment to problem solving across organization boundaries.

For single-use systems, supply chain excellence requires a commitment to problem solving across organization boundaries.

Using closed systems opens up many new possibilities for how facilities are designed and operated and may also present lower risk to the operation and, ultimately, the product.

Maintaining flexibility in biopharmaceutical manufacturing can deliver positive results.

Additional challenges to the new cleanroom paradigm from concurrent multiproduct manufacturing of bulk drug substances in a controlled non-classified (CNC) ballroom environment.

Benchmarking can be a useful tool to improve manufacturing practices.

Improvement strategy should be linked to business strategy.

Is process-centered organization in biopharmaceutical manufacturing a stepping stone or a stumbling block?

A one-day sign off for batch records is considered a best practice in the industry.

Leading industry collaborators outline top 10 best practices for human error reduction.

A closer look at elastomer changeout times provides one example of using industry knowledge to improve operations and cost.

Introducing a new way to think about sharing information in a patent-driven industry.
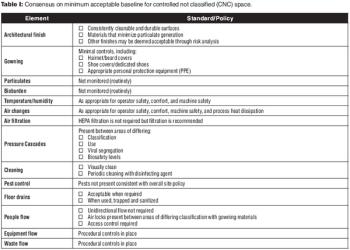
The authors re-examine environmental controls in the context of technical advances in manufacturing.
Published: April 1st 2014 | Updated:

Published: March 1st 2014 | Updated:

Published: November 1st 2013 | Updated:

Published: June 1st 2013 | Updated:

Published: May 1st 2013 | Updated:

Published: April 1st 2013 | Updated: