
Seattle Genetics could potentially receive approximately $500 million in fees, milestones and royalties.

Seattle Genetics could potentially receive approximately $500 million in fees, milestones and royalties.

Industry experts discuss the implementation of QbD and PAT tools in biopharmaceutical manufacturing.

Increased manufacturer outsourcing requires clear policies and written agreements with CMOs.

The EMA's Committee for Medicinal Products for Human Use has recommended granting of marketing authorizations for the first two monoclonal antibody biosimilars.
A Q&A with SCHOTT Pharmaceutical Systems and West Pharmaceutical Services

Bills to regulate drug compounding and establish a national track and trace system face political and policy differences.
An integrated focus on product design, development, operation, and control.


Maintaining flexibility in biopharmaceutical manufacturing can deliver positive results.

Rita Peters, Editorial Director for Pharmaceutical Technology and BioPharm Inernational talks with Mani Krishnan, director of systems at EMD Millipore live at Interphex 2013 about single-use technology. In this interview they discuss areas of growth in single-use technology as well as reservations that some companies have to switching to single-use systems Krishnan identifies a few technology advances that are overcoming these objections as well as decision-making strategies for facilities considering a switch to a single-use system.

Editorial Director Rita Peters interviews Alan Fisher of Dycem about risk management is applied to contamination control for pharmaceutical manufacturing

Project will optimize expansion and harvest of human cells in bioreactors.

New Center for Pharmaceutical Advancement and Training increases number of experts and available tools in Sub-Saharan countries.

While there are those who want combination products to be controlled by a centralized pharmaceutical-type approval system, the majority of the medical technology industry wants to retain a decentralized device-focused approach.

The author discusses issues related to the supply of soft parts in the biopharma industry.

BioPharm International spoke with INTERPHEX 2013 conference-session presenters to gain insight on trends in facility and process design.

Additional challenges to the new cleanroom paradigm from concurrent multiproduct manufacturing of bulk drug substances in a controlled non-classified (CNC) ballroom environment.

BioOutsource, a contract testing services provider to the biopharmaceutical industry, has opened a new facility in Cambridge, Massachusetts. The facility marks the company's first North American offices, which were opened in response to increasing demand for the company's bioanalytical and biosafety services, particularly in the field of biologic and biosimilar characterisation.

Third compounding pharmacy recalls products due to FDA inspection.

Unique technology expands Entegris? fluid-sensing and control offering.

Catalent Pharma Solutions has acquired a license to market Redwood Bioscience 's proprietary SMARTag precision protein-chemical engineering technology.

Astellas and Ambrx have entered into a collaboration to discover and develop novel antibody drug conjugates (ADCs) for an undisclosed number of targets in oncology. ADCs enable targeted delivery of drugs to the target tissue.

Teva and Lonza have announced that their joint venture will continue to develop, manufacture and market affordable, efficacious and safe biosimilars.
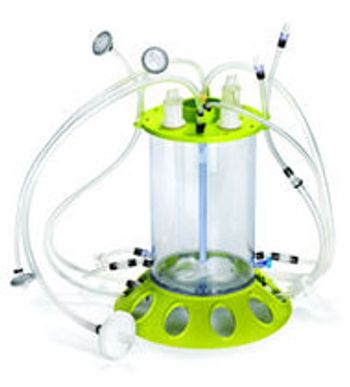
Review the process-design space and scalability of a single-use, stirred-tank bioreactor.

Jerold Martin considers the types of tubing available to the industry and how to make an informed selection.