
The sterile injectable manufacturer Ben Venue decides to end production despite efforts to remediate cGMP violations.

The sterile injectable manufacturer Ben Venue decides to end production despite efforts to remediate cGMP violations.

Advanced Biosciences Laboratories will support the development and production of a novel Shigella vaccine candidate for PATH.

The first part of CPhI's Annual Expert Industry Report examines ADCs, single-use technology, and regulatory failure.

GlaxoSmithKline?s sale of its thrombosis brands is part of a focus on its late-stage pipeline.

Fujifilm Diosynth Biotechnologies? new mammalian cell-culture facility uses primarily single-use technologies.

Manufacturing standards are considered key to preventing drug recalls and shortages.

The authors review the angiopoietin pathway as an alternative for safer and more efficacious anti-angiogenic therapeutics.
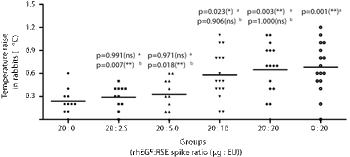
The correlation between limulus amebocyte lysate (LAL) assay and rabbit pyrogen test (RPT) targeting recombinant human epidermal growth factor (rhEGF) as active molecule was assessed.

ATMI has invested in sterile-connector and sterile-fill technology developed by Medical Instill Technologies.

GE Healthcare Life Sciences announced that it will build a KUBio modular biopharmaceutical factory in China for JHL Biotech.

FDA Issues Draft Guidance on Patient Counseling Info for Labeling

Following passage in the California Assembly, the California State Senate passes legislation to specify requirements for dispensing biosimilars.

Mike Jenkins, general manager of Catalent Biologics discusses the evolving landscape of the biologics market and hurdles faced in the development and manufacture of these innovative products.

Restricted access barrier systems (RABS) maximize product control but minimize operator interaction in aseptic manufacturing.

Are strategic partnerships in clinical research a model for CMC services?

Novasep's Sius single-use tangential flow filtration skid offers a 100% single-use TFF solution.

New FDA supply chain policies aim to strengthen inspection and oversight processes.

As the complex requirements of manufacturing biologics are manifold, it is important that biomanufacturing companies adopt quality-by-design principles.

Continuous countercurrent tangential chromatography (CCTC) is a column-free process that provides a scalable, disposable, and cost-saving alternative to column chromatography.

EMD Millipore and PharmaCell have entered into a collaboration to develop optimized large-scale expansion and harvest of HepaRG cells using bioreactor technology.

FDA receives adverse event reports related to calcium gluconate infusions.

A proposed new guideline provides a global policy for limiting metal impurities qualitatively and quantitatively in drug products and ingredients.
Uwe Gottschalk, vice-president of purification technologies, Sartorius Stedim Biotech, discusses specific challenges in protein purification.

Advances in techniques and single-use systems are revolutionizing vaccine manufacturing.

The author discusses HTST pasteurization and UHT sterilization.