
A novel approach to sterile drug product manufacturing uses a single-use assembly in a multi-product final filling suite with isolator technology.

A novel approach to sterile drug product manufacturing uses a single-use assembly in a multi-product final filling suite with isolator technology.

An environmental study of single-use process technology for biopharmaceutical manufacturing offers a comprehensive examination of environmental impacts across the full process train using lifecycle assessment.

FDA clarifies recommendations for injectable drug products packaged in vials and ampules.

Walker Barrier Systems builds eight mobile clean rooms for the Texas A&M Center for Innovation in Advanced Development and Manufacturing.

Plastic pump chamber is a cost-effective alternative for biopharmaceutical applications.

ASI Life Sciences introduced the DHX Disposable Heat Exchanger and the imPULSE MDS mixing, docking, and shipping system for single-use biopharmaceutical processes.

HHS plan makes progress in ensuring availability of safe vaccines.

Precalibrated instruments deliver accurate readings and withstand rigorous use.

Research uncovers trends and factors affecting the pre-packed disposable chromatography columns market for downstream bioprocessing.

Experts discuss the advances in single-use technology for downstream bioprocessing applications.
Adopting single-use systems has implications for manufacturing execution systems (MES), process data-analytics tools, and scheduling. Enterprise resource planning and MES must be integrated using standards such as ISA-95. Master and electronic batch records must be designed to capture the details of single-use component assembly to enable traceability.

Researchers are using current understanding of the lyophilization process to predict performance on many levels during both process development and manufacturing.

ALpHA G Capsule Filter for Single-Use Systems

Manufacturers are taking measures to comply with new package safety rules.

For single-use systems, supply chain excellence requires a commitment to problem solving across organization boundaries.
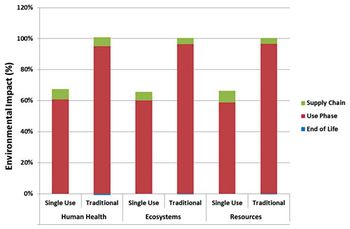
An environmental study of single-use process technology for biopharmaceutical manufacturing offers a comprehensive examination of environmental impacts across the full process train using lifecycle assessment.

A novel approach to sterile drug product manufacturing that uses a single-use assembly in a multi-product final filling suite with isolator technology offers benefits of efficiency and flexibility.

FDA provides recommendations for submitting analytical procedures and methods validation data.

Thirteen companies are accepted for participation in the supply chain program.

Tandem Diabetes Care expands its voluntary recall of select lots of insulin cartridges used with t:slim insulin pump.

EMA releases an update on its flu vaccine guidance.

Studies show diminished cell growth properties associated with some biocontainer or bioreactor films.

With numerous biologics set to come off patent soon and the percentage of new therapeutics based on biomolecules growing, demand for contract manufacturing in the biopharma space is heating up.

A packaging defect has led to Merck voluntarily recalling all lots of Liptruzet tablets in the United States.

Key considerations for implementing single-use components or platforms when moving from research to process development.