
Correct organization and appropriate methods for demonstrating biosimilar comparability are important for supporting regulatory filings.
Thomas A. Little, PhD, is president, Thomas A. Little Consulting and BioAssay Sciences, 12401 North Wildflower Lane, Highland, Utah 84003, USA, drlittle@dr-tom.com.

Correct organization and appropriate methods for demonstrating biosimilar comparability are important for supporting regulatory filings.

Developing analytical methods and bioassays is necessary from early on in the drug development life cycle.

Developing an effective bioassay is crucial for determining the potency of a drug substance or finished drug product. This article gives an overview of how to avoid most problems associated with correct bioassay development.
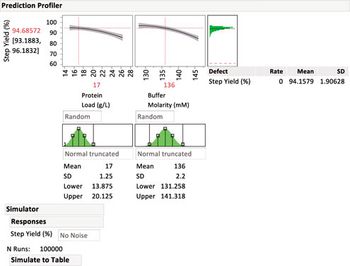
Statistical methods to identify critical process parameters and critical material attributes-and approaches to control them-are needed to protect drug product and drug substances.
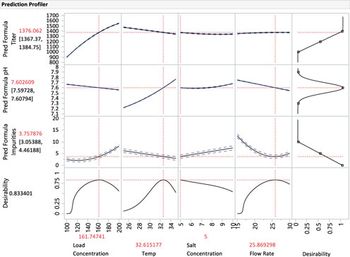
This article examines how process characterization is crucial to process understanding and then applying that understanding to controlling a process.
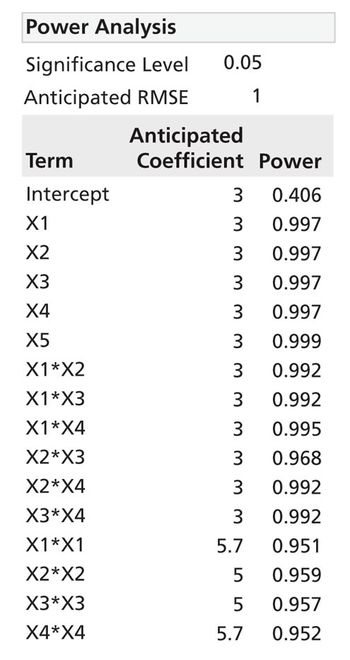
Process characterization and model building are essential skills and are required for modern drug development.
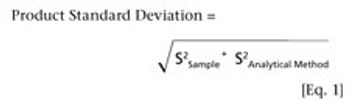
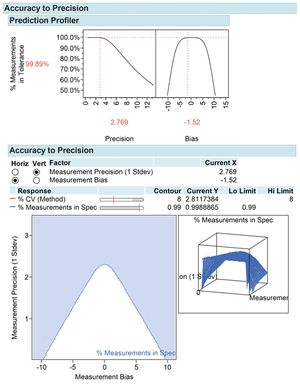
Knowing how method performance impacts out-of-specification rates may improve quality risk management and product knowledge.
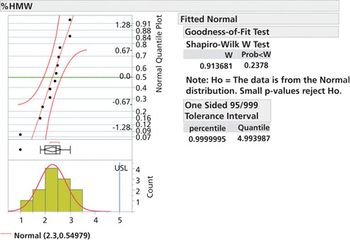
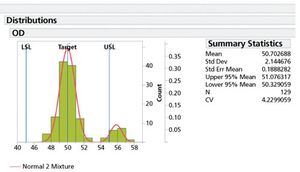
Specification limits should be set early in drug development and refined in later phases as data becomes available.
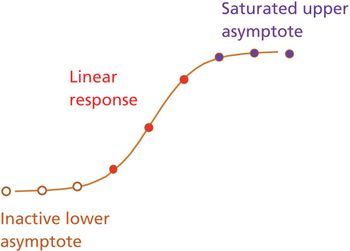
The author describes common components of a relative potency bioassay and provides a framework for assay development, calculation, and control.
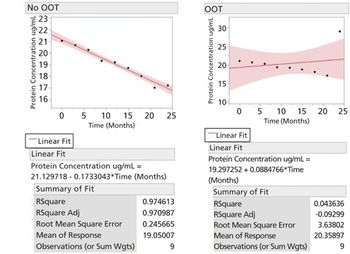
This article defines the concept, justification, and method of removal of out-of-trend points in stability modelling and shelf-life prediction.
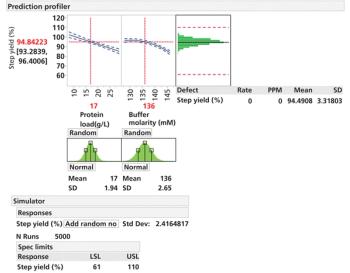
An approach to small-model generation and calibrating small-scale models to reliably predict performance at scale is presented.
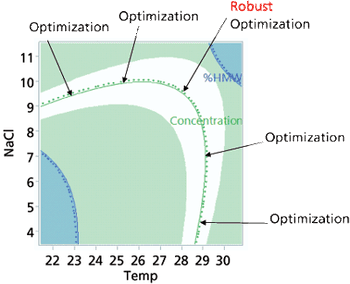
Approaches to the generation of process models, optimization techniques, and application of a design space are explored.
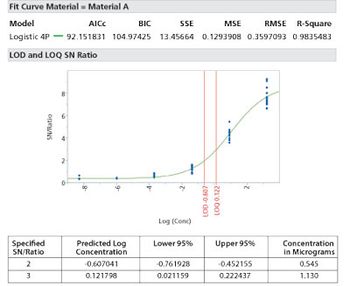
Care needs to be made to match the method of limit determination to the analytical method.
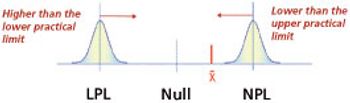
Understanding the influence of change events on product performance is a necessity to routine drug development, transfer, and validation.
The ability to define a scientifically justified and statistically sound sampling procedure is a fundamental skill in modern systematic drug development.
Design space generation is encouraged in new product development.
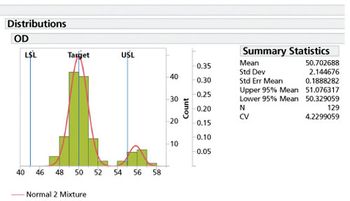
Characterization of stability performance provides a clear, statistically defendable method for determining accelerated stability.
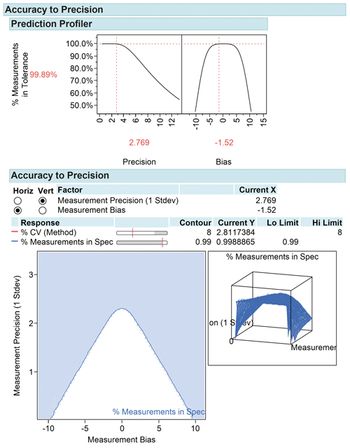
Design of experiment is a powerful development tool for method characterization and method validation.
Variation understanding and modeling is a core component of modern drug development.
Knowledge of product or process acceptance criterion is crucial in design space.
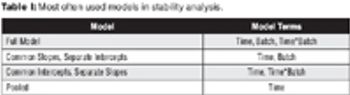
The author discusses the need for stability analysis.
Quality risk management is an essential element of every aspect of drug development and manufacturing throughout the product lifecycle.

A 10-step systematic approach to analytical method development and validation can improve the quality of drug development.
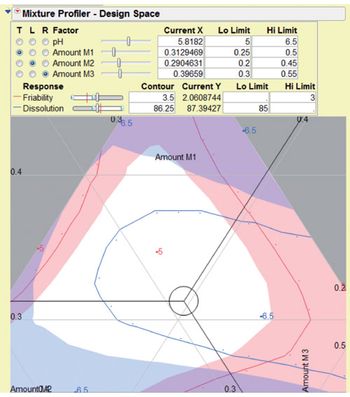
Harmonized regulations call for a risk-based and systematic approach to evaluating and selecting CPPs.

Published: August 1st 2021 | Updated:
Published: March 1st 2014 | Updated:
Published: December 1st 2013 | Updated:
Published: September 1st 2013 | Updated:
Published: May 1st 2013 | Updated:

Published: July 1st 2013 | Updated: