
Virosomes present novel drug-delivery vehicles with distinct advantages over liposomes.

Virosomes present novel drug-delivery vehicles with distinct advantages over liposomes.
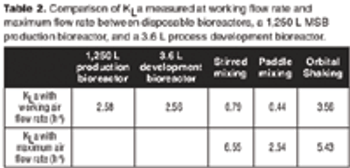
A case study to compare the performances of several types of mixing in disposable bags with stainless steel bioreactors.
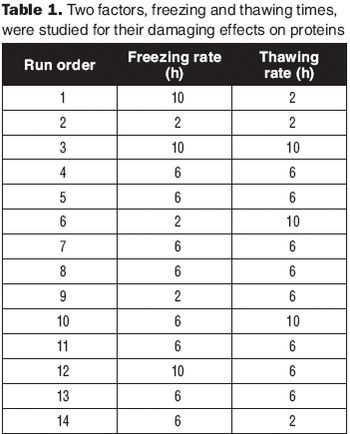
Apply a DoE strategy to test several formulations in parallel.
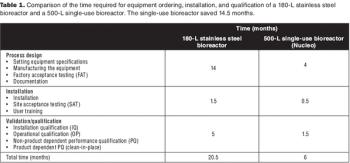
Sanofi Pasteur's disposables implementation plan is part of a larger evaluation of technology innovation. Here's how they approach it.

ATMI, Inc. has acquired the Belgian biotechnology firm Artelis SA, which offers technologies for cell culture research and manufacturing scale-up.

A case study evaluates the performance, control of operations, productivity, and cost savings of a single-use system.
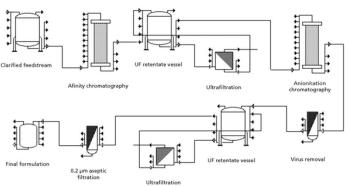
Single-use TFF offers the greatest savings in clinical and contract manufacturing, where the scale is low and changeovers are frequent.
Switching to single-use bioreactors can have financial and performance benefits.

BPSA eases confusion over extractables and leachables testing through guides.
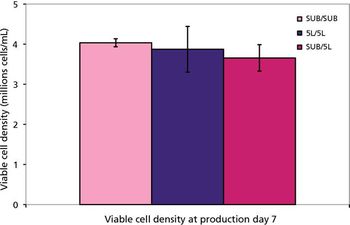
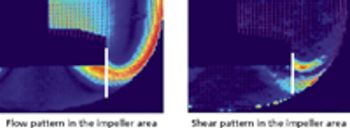
Despite different material, agitation, and aeration, the performance of the disposable bioreactor is similar to that of stainless steel bioreactors.
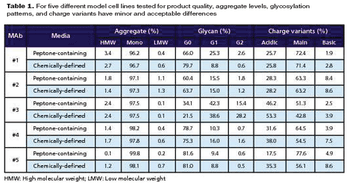

Release testing involves both standard potency assays and unique assays (particle size, NA activity) developed to ensure the physical, chemical, and biological stability of this type of vaccine.

Adjuvant activity can be greatly improved by appropriate formulation of cytosine-phosphorothioate-guanine oligodeoxynucleotides (CpG ODNs).
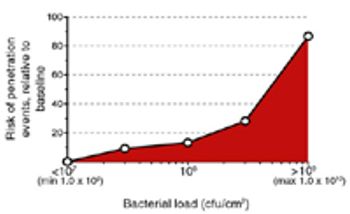
Filterability and bacterial retention must be verified very early in process development to ensure successful sterilizing filtration validation.

Adjuvant activity can be greatly improved by appropriate formulation of cytosine-phosphorothioate-guanine oligodeoxynucleotides (CpG ODNs).


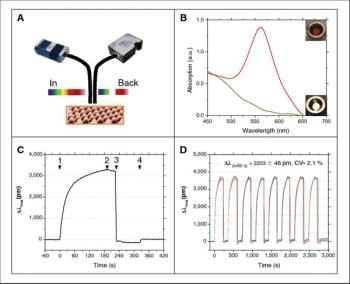
Use it label-free, or add labels to detect contaminants in solution.
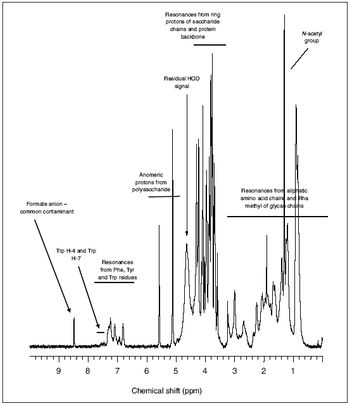
Simple methods can characterize polysaccharide vaccines and recombinant cytokines at high resolution.
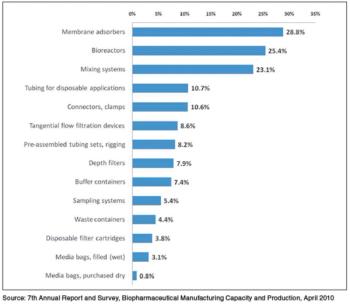
Although single-use systems are widely used in upstream unit operations, their acceptance in downstream processes has been slow.

How a Big Pharma company tackled the move to disposable bioreactors.

New Brunswick Scientific, an Eppendorf Company (Edison, NJ), and Pall Corporation (Port Washington, NY) have entered into a product development and marketing agreement to create and supply new disposable bioreactor systems.


Vetter held a groundbreaking ceremony for its new facility in Ravensburg.

International outsourcing and rising theft spur regulatory action and manufacturer oversight.