
An automated analytical method determines the purity of chromogenic, fluorescently tagged proteins or metal-bound proteins.

An automated analytical method determines the purity of chromogenic, fluorescently tagged proteins or metal-bound proteins.

Industry players form Allotrope Foundation to solve analytical data management problems.

Data analytic strategies can help companies capitalize on personalized medicine.
Light scattering analysis combined with more rapid size exclusion chromatography improves protein characterization.

Genia's DNA-sequencing platform will strengthen Roche's pipeline.

TOC Analyzer Increases Productivity
Handheld Analyzer Improves Accuracy

SEC-MALS Detector for UHPLC
Characterization of stability performance provides a clear, statistically defendable method for determining accelerated stability.

The ?DAWN can be attached to any UHPLC system to determine molecular weights and sizes of proteins.

The Sievers M9 TOC Analyzer enhances productivity.

Line of Environmental Measurement Products

UPLC Separation Intergrates into Mass Spectrometers
USP evaluates raw materials used in the chemical synthesis of peptides.
High-Performance Separation-Electrospray Ionization System

Mass Spectrometer Enhances Performance in Protein Analysis
Liquid Chromatography Column Separates Glycans

Aggregation System Improves Analysis of Sub-Visible Particles
USP evaluates quality attributes for synthetic peptides.
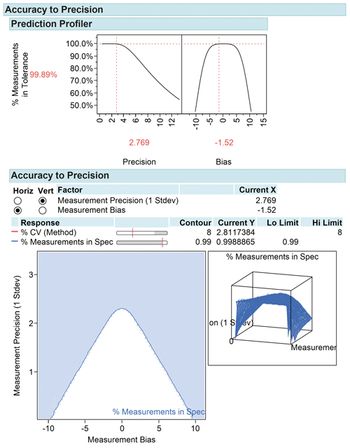
Design of experiment is a powerful development tool for method characterization and method validation.

FDA provides recommendations for submitting analytical procedures and methods validation data.
Advances in instrumentation, software, and methodologies provide more information than ever on the higher-order structure of proteins.

Accurate protein concentration results can be obtained using standard spectrophotometers and commercially available short path length absorption cells.

USP announced the approval of General Notices section 5.60.30 Elemental Impurities in USP Drug Products and Dietary Supplements with an official date of Dec. 1, 2015.
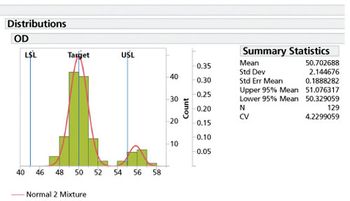
Variation understanding and modeling is a core component of modern drug development.