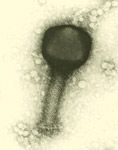
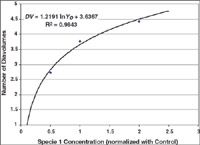
For decades now, it has been said that "the process is the product" for biologics. Great care and consistency must be applied in their upstream manufacture-during fermentation, harvest, and early purification-to preserve their complex structure, which confers their activity and specificity. As the product moves to late-stage purification, however, the relative concentration of impurities and altered product forms is diminished. Also, the final dosage form of most large molecule biopharmaceuticals is the relatively simple liquid formulation of parenteral dosage form. In contrast, manufacturing the solid dosage forms common for small-molecule drugs involves more complex processes, such as mixing dry powders, granulation, manufacturing controlled-release matrices, and tableting.