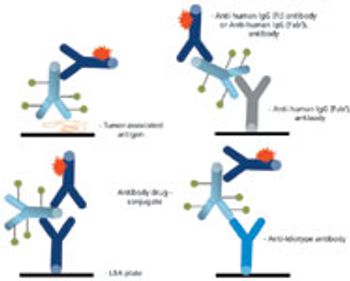
The complex structure of ADCs necessitates different analytical strategies than those for either small molecules or unconjugated monoclonal antibodies.

The complex structure of ADCs necessitates different analytical strategies than those for either small molecules or unconjugated monoclonal antibodies.
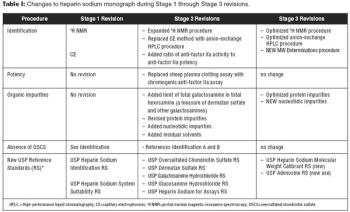
USP optimizes identification tests and impurities procedures.
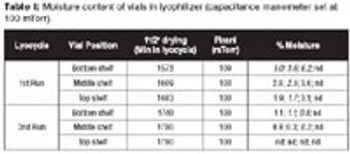
Using an alternate moisture-generation method may provide more accurate data for regulatory submissions.
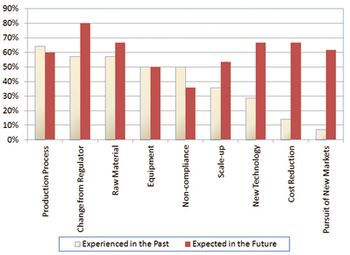
MIT survey results address product and site characteristics that statistically correlate with quality performance.

Using a competency-based approach to effectively train biopharmaceutical industry staff.
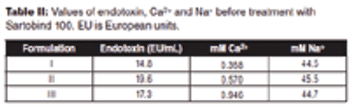
The authors describe a simple method to remove endotoxins from highly viscous formulations.

Recently developed immunoassay technology platforms reduce sample volume requirements and improve cycle times.
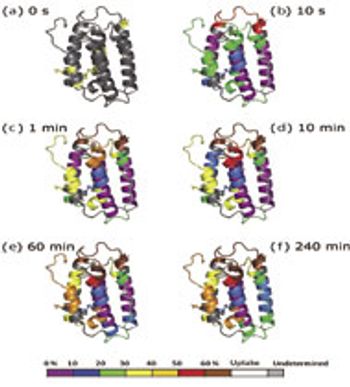
In HDX studies, data are produced across multiple time points, multiple species, and with replicates.
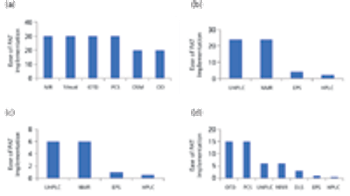
The authors review the various analytical methods that can enable use of PAT.
Current expectations in bioprocessing and a framework for using NMR to enhance a QbD approach.
Members from an ASQ working group provide analytical methods to enable PAT.

Unnecessary analytical testing can lead to unnecessary costs.
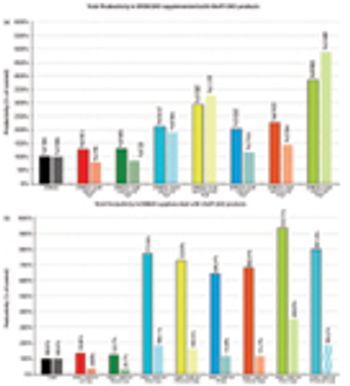
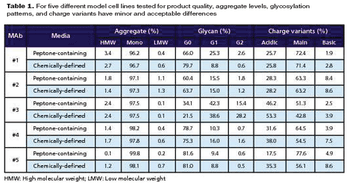
Cell-line specific complex media supplements combine chemically defined media additives into a single supplement.
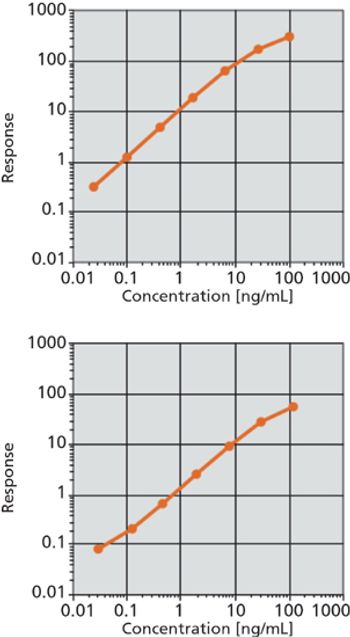
The authors discuss a new, rapid immunoassay for the detection of biomarkers.

Future sponsor-contract provider relationships will require more integration.

Rather than seeking a single indication for one large group, the orphan-drug approach segments the market for a drug more minutely.

During this podcast, we will discuss the causes and implications of Adventitious Agent Contamination for pharmaceutical and biopharmaceutical manufacturers. We will discuss ways in which Lancaster Laboratories is helping manufacturers minimize the risk for this contamination to avoid production delays.
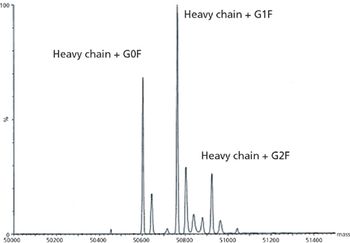
The author describes techniques that can be used to provide the analytical data required by ICH Q6B for characterization of monoclonal antibodies.
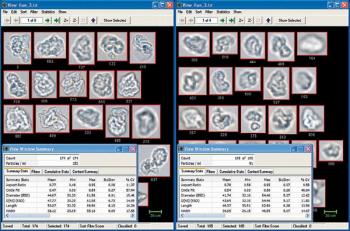
Overcoming limitations of volumetric techniques and detecting transparent particles.
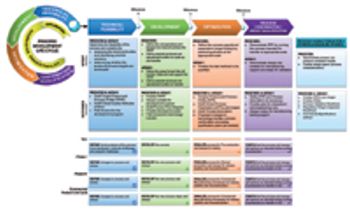
Achieving multiproduct development within shortened timelines.
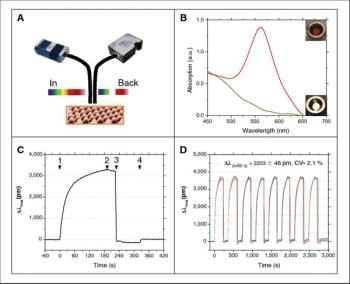
Use it label-free, or add labels to detect contaminants in solution.
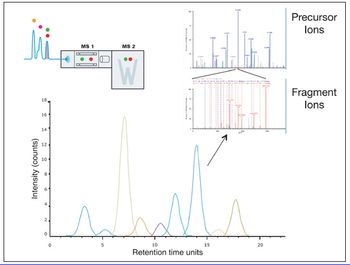
By providing information on the relative accessibility of locations within a protein, HDX by mass spectrometry opens new windows into the higher order structure of biomolecules.
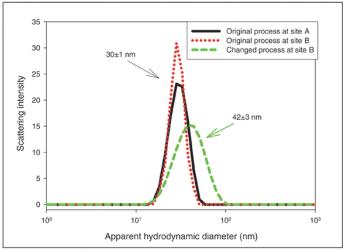
Spectroscopic methods such as circular dichroism can detect subtle differences in higher order structure before and after changes in process and formulation.
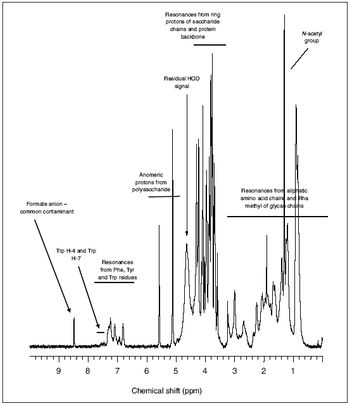
Simple methods can characterize polysaccharide vaccines and recombinant cytokines at high resolution.