
Higher cell densities, greater demand for high-performance viral clearance, and desire for large-scale single-use technologies are driving development of filtration technologies.

Higher cell densities, greater demand for high-performance viral clearance, and desire for large-scale single-use technologies are driving development of filtration technologies.

The rapid testing of biologic raw materials can lead to greater efficiency.
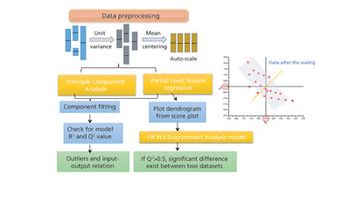
The authors review major developments in use of MVDA in bioprocessing applications.

This article presents first-hand perspectives from industry users to suppliers of single-use sensors.
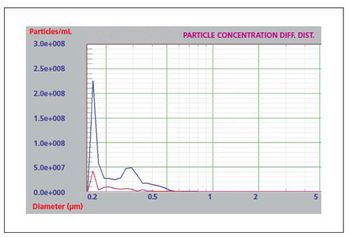
Liquid particle counters are ideal for protein aggregation studies.
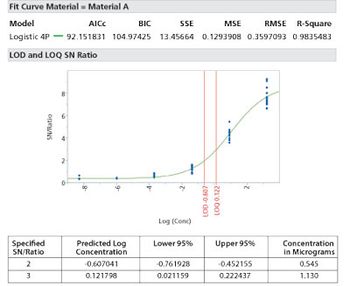
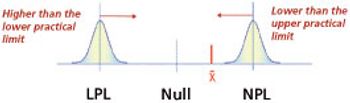
Care needs to be made to match the method of limit determination to the analytical method.

The agency outlines recommendations for the development and submission of near infrared analytical procedures.
The thorough analysis of a therapeutic protein product’s propensity to aggregate may be a necessary step in the prevention of a cell-mediated immune response.

MedImmune will provide funds and access to monoclonal antibodies to seven postdoctoral associates for the creation of protein measurement and characterization tools.
As ADCs move through the drug-development process, different analytical methods are often required.
Understanding the influence of change events on product performance is a necessity to routine drug development, transfer, and validation.
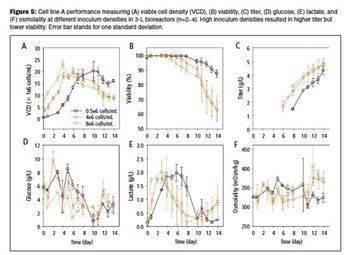
The use of commercially available media to achieve high titer in early process development is discussed.

The author discusses the various ways in which a quality-by-design program can enhance the extractable and leachable assessment of a drug product.

New drugs submitted for approval in Europe have 18 months to comply with new elemental impurities guidelines.

A new study conducted by the National Institutes of Health found that a certain vector used in gene therapy (and its insertion site in the genome) may be associated with an increased risk of liver cancer.

USP establishes Jan. 1, 2018 as the implementation date for its elemental impurities guidelines for existing drugs.
Understanding and preventing protein aggregation is crucial to ensuring product quality and patient safety.
Ligand-binding assays are fundamental to characterizing biosimilars.

The authors review efforts to limit polymer degradation without significantly impeding cell growth.

MedicalRF.com/Science Photo Library-ANDRZEJ WOJCICKI/Henrik Jonsson/Stocktrek Images; Dan Ward

The use of orthogonal methods to SEC is discussed and examples are presented showing how analytical ultracentrifugation, AF4, and SEC compare in aggregate quantitation.

Defining critical parameters and processing large quantities of data can be a challenge.
The ability to define a scientifically justified and statistically sound sampling procedure is a fundamental skill in modern systematic drug development.
A quality-by-design approach that defines potential viral contaminants of source materials can be used to achieve viral clearance.

Adding light scattering to size-exclusion chromatography (SEC) can maximize the benefits of SEC.