Biopharmaceutical Analysis
Latest News


Surface plasmon resonance is helping define bispecific antibodies, the next-generation of biopharma therapeutics.
Dynamic light scattering techniques can monitor viruses and virus-like particles in their native state.

The National Biologics Manufacturing Centre will provide companies with open access to bioprocessing facilities and expertise to expedite low-risk market entry of complex biologics.
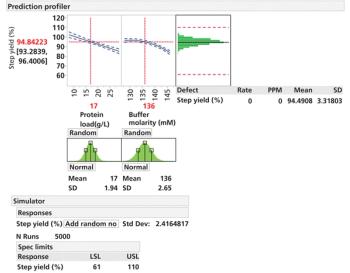
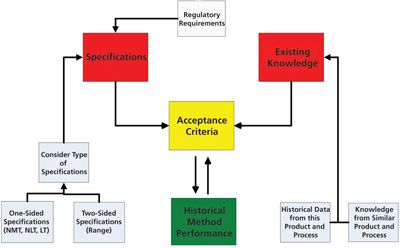
An approach to small-model generation and calibrating small-scale models to reliably predict performance at scale is presented.

Wilden’s Saniflo Hygienic Series pumps use a new, energy-efficient air distribution technology.
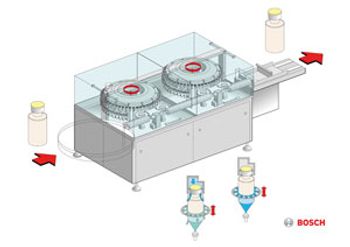
The RAN 3080 exterior washing machine from Bosch cleans filled and closed vials, ampuls, and cartridges using a high-pressure process and special transportation system.

Three case studies illustrate some analytical methods important for stability testing.

imagewerksBiophysical binding studies utilizing surface plasmon resonance, biolayer interferometry, isothermal titration calorimetry, or related techniques are ce

Sorendls/Getty ImagesTo maintain a state of control and comply with regulatory authorities, many pharmaceutical, biotech, and medical-device companies have adopted continued pro

Adents’ Pharma Suite serialization software features track-and-trace capabilities.

Ensuring data integrity involves effort on an individual and global basis.
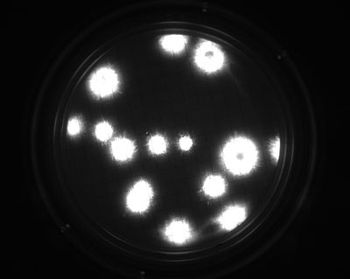
The rapid microbiological growth-based method represents an alternative for the quantification of contaminants in filterable products.

GEA's self-contained homogenizer is designed for laboratory applications, including cell dispersions.

Industry experts spoke to BioPharm International about the key considerations in the development of a drug-delivery device for a biologic drug, the importance of human factors engineering, the advantages of prefilled syringes, and the challenges in the manufacture of these devices.

A thorough cell-bank testing plan is necessary to certify the safety and purity of a resulting biopharmaceutical product.

The authors explore the use of statistical experimental design and multivariate analysis to develop a drug substance formulation matrix.

Malvern Instruments' biophysical characterization equipment will be installed in a commercial applications laboratory in San Diego, California.

The guidance provides recommendations for submitting analytical procedures and method validation data to FDA.

The collaboration will address the need for novel analytical approaches for the characterization of glycans.

Charles Rivers strengthens its endotoxin testing and bacterial identification detection capabilities with the addition of Celsis’ products.

The challenges and strategies of assessing and mitigating risk in biopharmaceutical manufacturing are discussed.

Advances in adventitious agent detection methodology are bringing benefits, but more work needs to be done.
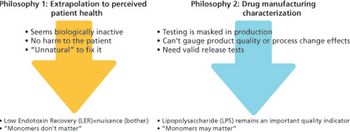
Low endotoxin recovery represents an opportunity to add value to the characterization of biologic drug products.
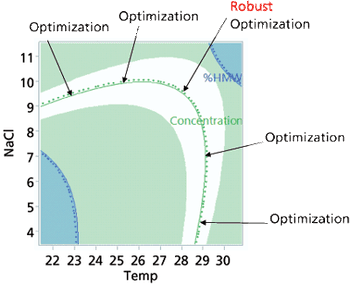
Approaches to the generation of process models, optimization techniques, and application of a design space are explored.