Review the importance of characterization studies during biosimilars development and related analytical methods.


Review the importance of characterization studies during biosimilars development and related analytical methods.
Knowledge of product or process acceptance criterion is crucial in design space.

A proposed new guideline provides a global policy for limiting metal impurities qualitatively and quantitatively in drug products and ingredients.

Viruses in animal-derived starting materials could contaminate biopharmaceutical final product. A rigorous testing strategy and removal methods are reviewed.
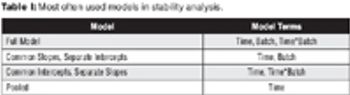
The author discusses the need for stability analysis.

The authors provide common misconceptions and key concepts behind reliability engineering.

Rita Peters, Editorial Director for Pharmaceutical Technology and BioPharm Inernational talks with Ferdinand Dabu, director of marketing at SGS live at Interphex 2013 about extractables and leachables. In this interview they discuss special considerations for testing for extractables and leachables for both small molecule and biological drug development.

Rita Peters, Editorial Director for Pharmaceutical Technology and BioPharm Inernational talks with Ferdinand Dabu, director of marketing at SGS live at Interphex 2013 about extractables and leachables. In this interview they discuss the key elements of an effective testing program to identify extractables from materials and leachables in drug products.

Rita Peters, Editorial Director for Pharmaceutical Technology and BioPharm Inernational talks with Ferdinand Dabu, director of marketing at SGS live at Interphex 2013 about extractables and leachables. In this interview they discuss new analytical testing procedures and instruments that are facilitating the testing processes or improving results.

Editorial Director Rita Peters talks to with Ferdinand Dabu Director of Marketing at SGS live at Interphex
The author describes a method to avoid protein aggregation when using light scattering systems.
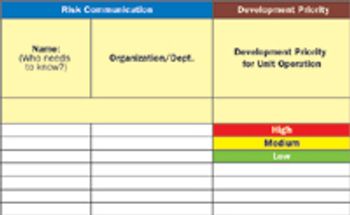
Quality risk management is an essential element of every aspect of drug development and manufacturing throughout the product lifecycle.

At Pittcon 2013, Bruker introduced a range of instruments and software updates including two LC-triple quadrupole (LC-TQ) mass spectrometers.

Rigaku Raman Technologies announced an updated version of the FirstGuard handheld Raman analyzer at Pittcon 2013.

As biopharmaceutical/pharmaceutical companies increase their development of biologic-based drugs, companies providing analytical instrumentation and laboratory testing goods and services are, in turn, offering improved tools for biologic characterization, biomanufacturing, and related testing.
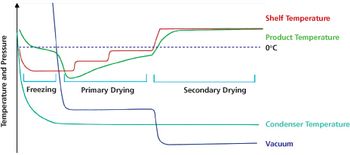
Optimized freeze-drying cycles can offer scientific and business advantages.

BioPharm International spoke with industry experts about the effect FDA's 2011 process validation guidance has had on industry.
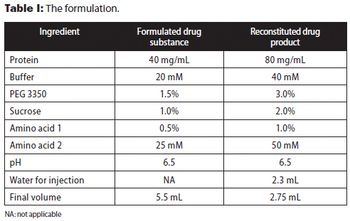
The authors present approaches used to reduce reconstitution time of a lyophilized high-concentration protein drug product.

Applying quality-by-design and process analytical technology facilitates process understanding and control of various operations in lyophilization.

NIBRT's Michael Lacey provides an overview of biopharmaceutical facility design and operation.

Project: transfer a manual concentration/diafiltration process for siRNA production.

FDA talks about the changing scope of regulatory science.

What the industry's future holds and what needs to be done to get there.

NIBRT's Ray O'Connor provides an overview of aseptic processing.

Preparation of biological samples for chromatographic analyses.