The development of mAb formulations poses challenges at the manufacturing, stability, analytical, and administration levels.

The development of mAb formulations poses challenges at the manufacturing, stability, analytical, and administration levels.

Interactions between biologic drug products and the components of prefilled syringes can cause protein aggregation, but there are alternative materials that can help mitigate this problem.

BioPharm International spoke with Trevor Marshall, director of enterprise systems integration at Zenith Technologies about automating processes in upstream processing.

The new guidelines will address bioanalytical method validation and biopharmaceutics classification system-based biowaivers.

The authors describe the qualification of an assay with applications for investigating functional comparability of an originator and biosimilar drug.

Laser-induced fluorescence, a rapid microbiology method for real-time airborne particle and microbial monitoring, enhances sterility assurance in pharmaceutical manufacturing.
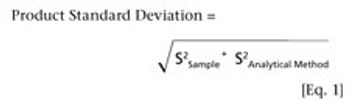
Knowing how method performance impacts out-of-specification rates may improve quality risk management and product knowledge.

Industry experts discuss best practices for selecting a separation technology.
This study offers a strategy for stabilization of biotherapeutics for long-term frozen storage in PBS-based formulations.

Model effectiveness is determined by the quality and composition of the data inputs.
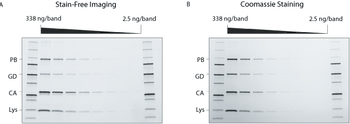
Innovations in electrophoresis and chromatography upstream of protein characterization can accelerate research.

The Chinese facility was cited for data integrity violations.

Experts discuss recent advances in cell viability testing methods in bioreactors.

Multiple methods are required for detecting and removing protein impurities.

A step-wise process is used to characterize glycans and understand the functioning of a molecule for biosimilar development.

NIST’s monoclonal antibody reference material can be used as a standard for biopharmaceutical analytical quality control.

Pressures to accelerate current and next-gen therapies are challenging traditional microbiological testing methods.
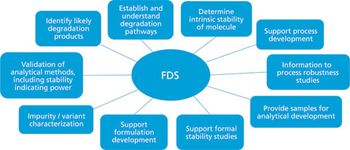
The author addresses critical issues to consider prior to performing forced degradation studies and provides best practice recommendations for these types of studies.

The assay, co-developed by Grifols and Hologic, will be used to test blood donations in the United States.
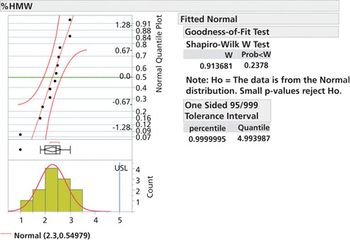
Specification limits should be set early in drug development and refined in later phases as data becomes available.

Advances in cell line engineering, process optimization, and in-vitro glycosylation are making a difference.

Investment at SGS’s Mississauga, Canada facility provides for analysis of molecular interactions in real time.
Data acquired from osmolality, glucose, and folic acid tests provides useful information for the specific identification of cell-culture media.
Testing product and process intermediates alone is helpful, but does not provide a complete solution to viral safety. This article proposes integrated solutions for systemic and proactive viral risk mitigation.

The agency publishes draft guidance on assay development and validation for immunogenicity testing.