
How statistical methods and novel indices can be used to monitor and benchmark variability, to guide continuous improvement programs.

How statistical methods and novel indices can be used to monitor and benchmark variability, to guide continuous improvement programs.
An increase in biologics raises awareness of particle generation and its role in negative patient outcomes.

Operated by BioOutsource, Sartorius’ subsidiary, the Glasgow, UK-based service center will offer physicochemical properties and structural attributes testing and allow clients to perform structural and functional analyses in parallel.

Can bioprocessing runs be consistently replicated in an inherently variable production environment?

This article provides an overview on important aspects related to bracketing strategies in Japan.

Endress+Hauser’s Memosens CPS171D pH electrode for hygienic and sterile biopharmaceutical processing provides stable measurements even after multiple cleaning and sterilization cycles.

Chromatography modeling can enhance bioprocessing efficiencies.
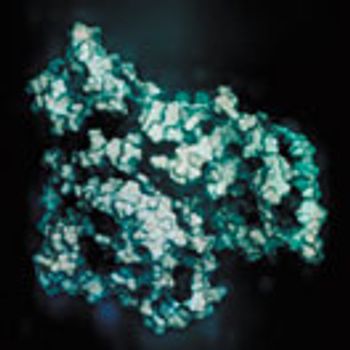
FTIR can successfully measure key characteristics of therapeutic proteins in a single step.

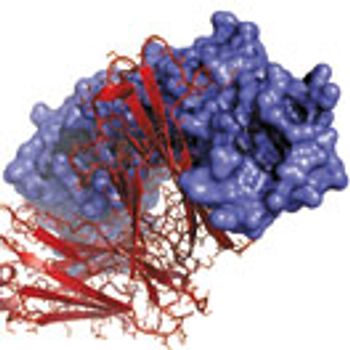
The complex nature of biologics adds additional CQAs that must be determined to ensure the safe development of biologics
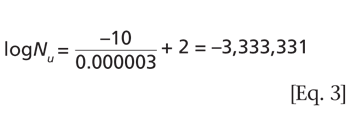
Understanding the purpose of the biological indicator can guide the development of an effective sterilization process.

The agency cited Morton Grove Pharmaceuticals for inadequate quality control procedures.

The decision to use disposable bioreactors is now driven by commercial rather than technological considerations.
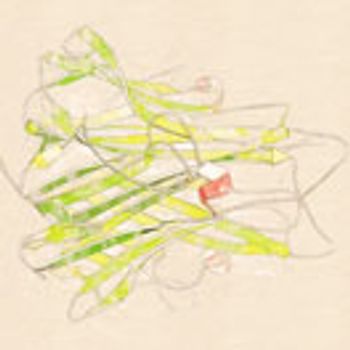
Manufacturing for originator molecules is restricted by regulations, but drug makers can exploit newer technologies for the manufacture of biosimilars.

Although best practices are key, advances in integrated informatics platforms and automation can make it easier to ensure data integrity and improve overall laboratory efficiency.
Process characterization and model building are essential skills and are required for modern drug development.

A multi-pronged approach to raw materials testing can help mitigate the risk of future contamination events.

Despite limitations, mass spec is having an impact on biologic drug development and manufacturing.

Extraction studies demonstrate approaches for evaluating single-use bio-pharmaceutical manufacturing materials.
The objective of this study was to assess the impact of manufacturing-scale, freeze-thaw conditions on aggregation and subvisible particle formation of a monoclonal antibody solution (mAb-A; IgG1) using a small-scale model.

PAT, quality by design, process controls, and analytical advances for small- and large-molecule drugs are on agenda for IFPAC 2017.

The company released the Biopharma Compass 2.0 software, which automates workflows for high-resolution mass spectrometry.
Isothermal titration calorimetry and differential scanning calorimetry are valuable tools that can help accelerate drug development.
This study aims at understanding the differences between porcine and bovine trypsin from both pancreatic and recombinant origins.

This report describes the first known attempt at quantifying the success of such processes in inducing nucleation on a 56–m2 freeze dryer operating at a load of 195,960 vials.
Understanding of endotoxin assays and a range of detection technologies are essential for effective testing.