
The ready-to-use assays are specifically designed for companies looking to characterize their biosimilar products in the pipeline.

The ready-to-use assays are specifically designed for companies looking to characterize their biosimilar products in the pipeline.

A new study concluded although some mAb products have heterogeneous variants, these charge variants are associated with similar potency and pharmacodynamic profiles as originator molecules.

The company will showcase workflow solutions for sample prep and data analysis.
The authors describe the ways in which manufacturers can mitigate the risks related to the integrity of recombinant transgenes expressed in CHO cells.
The necessity to detach cells from a culture substrate during cell harvesting remains one of the most challenging steps in a cell-culture process.

Collaborative efforts are underway between suppliers and drug manufacturers to address raw material variability.

The advanced capillary electrophoresis system, Maurice, from ProteinSimple is used for the quantitative analysis of identity, purity, and heterogeneity profiles of biopharmaceuticals.

Researchers describe a new method to compare the higher-order structure of a reference biologic with its proposed biosimilar product candidates.

Time and sensitivity are essential for analytical technologies in all phases of biopharma development.

Understanding of the risks associated with FMEA is crucial in lot release testing.
The recognition that microbial artifacts are capable of modulating the mammalian immune system is an emerging view of biologic drug contamination control testing.
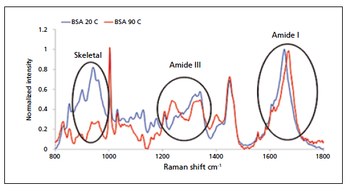
In this article, the author reviews some of the techniques that can yield valuable information on protein stability, focusing specifically on protein aggregation. Emphasis is placed on the enhanced information made available when technologies are used orthogonally, and the alignment of different approaches with specific stages of the biopharmaceutical development workflow.

The European Directorate for the Quality of Medicines & Healthcare announces the publication of a chemometric methods chapter in the European Pharmacopoeia.

The European Pharmacopoeia rewrites in its general chapter on Raman spectroscopy.

The authors explore the use of precipitation using polyvinyl sulfonic acid and zinc chloride in place of capture chromatography to reduce the cost of goods in the insulin manufacturing process.

The results of an industry workgroup’s examination of EMA’s guide on shared facilities are presented.
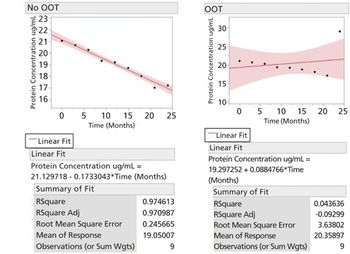
This article defines the concept, justification, and method of removal of out-of-trend points in stability modelling and shelf-life prediction.

The company’s new version of its glycan analysis tool will help investigators determine the relative abundance of individual N-glycan structures in small biopharmaceutical samples.

Protea will use Agilent’s mass spectrometry imaging technology to develop new methods to identify cell metabolites produced as a result of disease or drug exposure.

Subjective visual evaluation of freeze-dried products can be quantified through mechanical methods of characterizing the properties these materials.

The authors evaluate the SoloVPE technique as a replacement for nitrogen-based protein determination.

Expectations are high for rapid testing methods, but demonstration of comparability proves challenging.
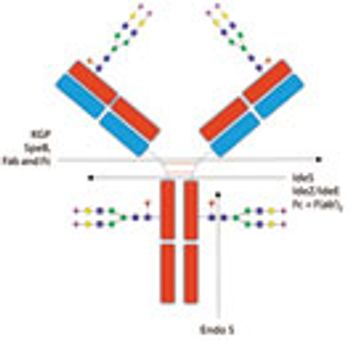
Advances in glycan analysis are enhancing biologics development and quality control processes.

Agilent Technologies and Thermo Fisher Scientific exchange instrument control drivers and software support.

FDA seeks feedback on possible analytical standards and approaches to optimize regulation of next-generation sequencing (NGS)-based in vitro diagnostic tests.