A UHPLC SEC approach for protein aggregate analysis of mAbs is presented.

A UHPLC SEC approach for protein aggregate analysis of mAbs is presented.

This study outlines methods for an alternative protein-polishing process for challenging proteins.

Bormioli Rocco Pharma, an Italian supplier of glass and plastic packaging, has launched its Delta-molded, type 1 glass vials in North America.
A case study demonstrates that affinity chromatography can offer efficiency and scalability for gene therapy manufacturing using viral vectors.

This review examines how microfluidics has been used in the formulation, preclinical, and clinical development of gene-delivery nanoparticles.

Along with particulate control and determination, speakers at the June conference in Rockville, Maryland, examined the role of protein aggregation and immunogenicity

Choosing a suitable material for fill/finish containers begins during the product development stage.

The authors review how media components modulate the quality of monoclonal antibody products

Steris added the Amsco 430LS and 630LS sterilizers to its line of medium steam sterilizers.

The expansion at its Lincolnshire, IL, facility will double the footprint of the site’s range of services, including microbiological evaluation, drug stability studies, and medical devices testing.

Amid debate about “fake news,” peer-review papers offer vital, objective insight.

Industry experts weigh in on best practices, challenges, and mutual recognition of cleaning validation standards.

Managing and prioritizing risk is essential to ensuring raw material quality. USP is developing new guidelines to make the work easier.

The author outlines an analytical strategy for establishing similarity in biosimilar development and approval.

Higher-flow peristaltic pumps from Watson-Marlow Fluid Technology Group are designed for upstream and downstream bioprocessing with single-use fluid path assemblies.

A Supreme Court decision and improvements in analytical processes may speed the biosimilar approval process.

A new probe developed by researchers at the University of Melbourne, Australia, would allow NMR to be used without the use of microwaves, and with smaller machines.

A new study predicts a potential disconnect between regulatory science and biotechnology product development, unless regulators scan the horizon for new developments and consider new potential risk pathways.
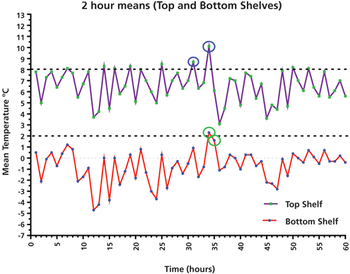
This column presents a data case study of a laboratory refrigerator and its qualification performance over five days, with important lessons for using average and individual results, as well as user requirements.

Method choice is crucial to when seeking answers to biosimilar characterization questions.

The authors summarize the current regulatory expectations regarding the number of PPQ batches required and provide potential approaches that can be used to determine and justify the number of PPQ batches.
While the measurement of the toxicity of leachables is not always a required parameter, the information collected during these studies could inform future bioprocessing runs.

Electronic systems can remove opportunities for individuals to make mistakes or to manipulate the data.

Optimize practices and meet requirements using electronic data integrity systems.
Short tandem repeat profiling is the most validated method to confirm cell line identity and avoid misidentified or cross-contaminated cell lines.