
The critical quality attributes of biotherapeutics must be monitored to ensure product safety and efficacy.

The critical quality attributes of biotherapeutics must be monitored to ensure product safety and efficacy.

This article explores lab data integrity violation trends, as well as a sampling of the latest technologies that can help avoid them.

Steve Hayward, product marketing manager at BIOVIA, Dassault Systèmes, discusses the importance of both technology and people in the modern laboratory.
The approaches for sample preparation of preclinical evaluation of safety and efficacy are addressed taking into consideration the shortcoming with the contemporary approaches.

The new Acquity Arc Bio System by Waters is specifically engineered to enable efficient transfer and improvement of bioseparation analytical methods.

Corning will feature its 3D bioprinting technology and tools to support 3D cell culture at booth #1029 during the SLAS 2018 conference on Feb. 3-7, 2018 in San Diego, CA.

The Fluent Gx Automation Workstation from Tecan is suited for a variety of clinical and regulated laboratory applications.

Formulatrix's Rover Laboratory Automation Platform to support sample preparation will be featured at booth #1335 during the SLAS conference in San Diego, CA, on Feb. 5-8, 2018.

Comecar will provide cell culture equipment for biopharmaceutical company CO.DON AG's new production site in Leipzig, Germany.

1CryoBio’s Flexiquot technology allows for the deep-freeze storage and handling of multiple aliquots in a single tube, winning it the CPhI Pharma Award 2017 for Excellence in Pharma: Analysis, Testing, and Quality Control.

Lonza Pharma & Biotech's's Modular Automated Sampling Technology (MAST) platform, which allows for the collection of up to 10 sterile sample sources for automated analysis in multiple analytical devices, won the CPhI Pharma Award 2017 for Excellence in Pharma: Bioprocessing.

The NGC Fraction Collector from Bio-Rad Laboratories allows researchers to choose how to collect and when to access their fractions for analytical or preparative chromatography applications.

Automated Control Concepts has launched Lab Owl bioreactor control system for labs using cell culture and fermentation applications.

The 2018 Short Course program at Pittcon, taking place Feb. 24–Mar. 1, will feature 23 new short courses geared towards laboratory professionals.

Conducting stability testing on APIs/finished drug product helps ensure shelf-life storage.
The authors present a robust and easy-to-implement chromatography column performance assessment method, called direct transition analysis (DTA).

BioTek Instruments has released a second edition of its BioSpa software that now offers users a simplified but effective interface for kinetic imaging or detection workflows.

SGS has introduced a Sanger sequencing service at its Glasgow, United Kingdom, laboratory to support genetic stability testing and perform identity testing on cell banks, plasmids, and viral seeds/vectors.

Biopharma majors are among the industry stakeholders who have commented and raised questions about FDA’s recently proposed draft guidance for analytical assessment of similarity in biosimilars.

Biopharma employees reveal employment objectives, opportunities, and frustrations.

A quality-by-design approach that implements PAT offers advantages in upstream cell-culture processing.

A roundtable Q&A with biopharma executives elucidates the challenges posed by single-use bioreactor bags in contributing to extractables and leachables in the biomanufacturing process.

MasterControl has added three new cloud-based analytical solutions to its MasterControl Version 11.7 software for clinical managers and regulatory/submissions professionals.
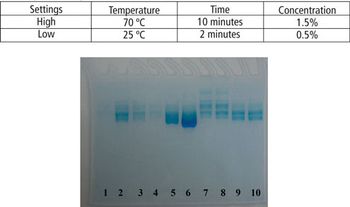
This article demonstrates that a modified SDS–PAGE can be easily used as a tool for quantifying the degree of protein degradation.
An updated version of Shimadzu’s LabSolutions analytical data system features an operating environment for complete data management and security in networked laboratories.