The approaches for sample preparation of preclinical evaluation of safety and efficacy are addressed taking into consideration the shortcoming with the contemporary approaches.
Department of Chemical Engineering, Indian Institute of Technology, Hauz Khas, New Delhi, India.

The approaches for sample preparation of preclinical evaluation of safety and efficacy are addressed taking into consideration the shortcoming with the contemporary approaches.
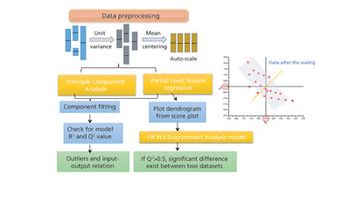
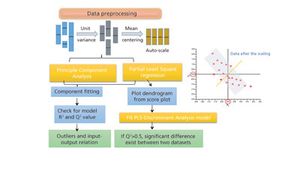
The authors review major developments in use of MVDA in bioprocessing applications.
Published: April 6th 2015 | Updated:
Published: February 1st 2018 | Updated: