
Computational fluid dynamics can resolve performance problems.

Computational fluid dynamics can resolve performance problems.
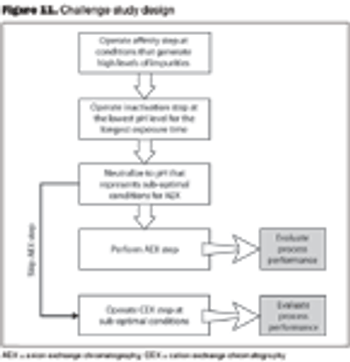
How to use risk assessment strategies to integrate operations.
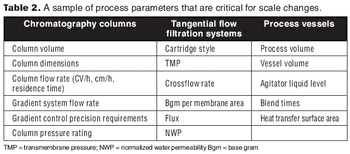
A process harmonization assessment can aid in smooth technology transfer by comparing data across equipment and sites.

The ability to deal with the complexity of the clinical supply process has shifted the balance of power to clinical supply chain specialists.
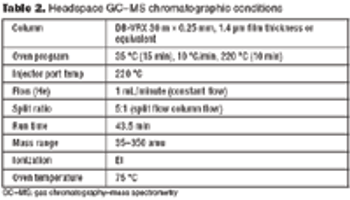
A systematic approach facilitates formulation component selection.
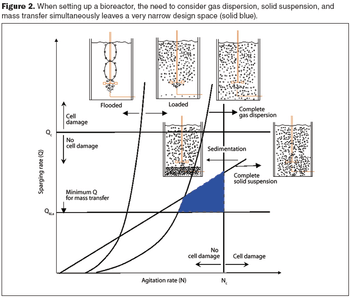
Computational fluid dynamics is a powerful tool to optimize processes.
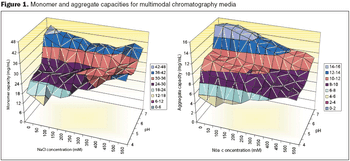
HTPD allows rapid screening of chromatographic parameters.

Humacyte, Inc. (Research Triangle Park, NC) and Xcellerex, Inc. (Marlborough, MA) have entered into an initial strategic collaboration for Xcellerex to develop a manufacturing process that will enable the production of Humacyte?s lead regenerative medicine product using Xcellerex?s XDR single-use bioreactor system in its FlexFactory.
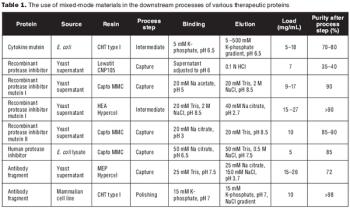
Salt-tolerant adsorption and unique selectivity are the major advantages of mixed-mode materials over single-mode resins.
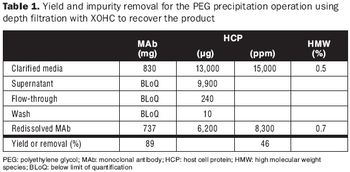
This alternative purification method to chromatography is readily scalable and fits a fully disposable downstream process.
To speed up the downstream process, you must get the right data, in the right amount, at the right time. Here's how.
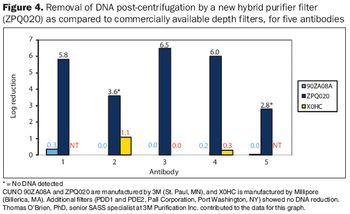
New technologies and adaptations of existing technologies can improve platform processes.
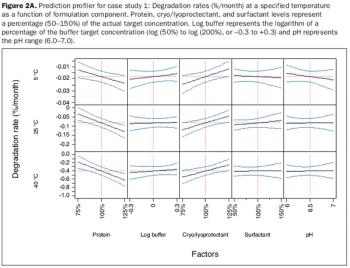
Design of experiments is a valuable tool for identifying aspects of a formulation that are critical to product quality.
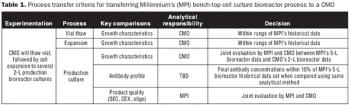
Sufficient process history is key to the rapid transfer of your process.

Sharing information is a critical part of security-whether we're protecting travelers from bombs or patients from adulterated medicines.
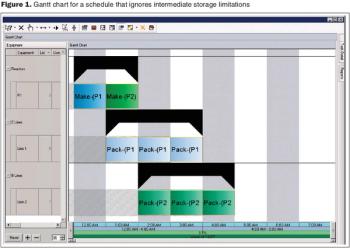
An algorithmic approach to fine tune facility design and predict capacity.

How this Big Pharma company successfully implemented disposable technologies in its manufacturing plant.
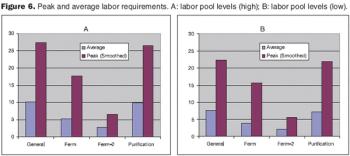
How to improve facility design and predict capacity.
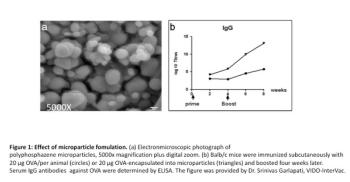
This article presents ongoing research at VIDO-InterVac for the development of safer and more effective adjuvants. These include adjuvants that stimulate innate immunity in the body and combination adjuvants.
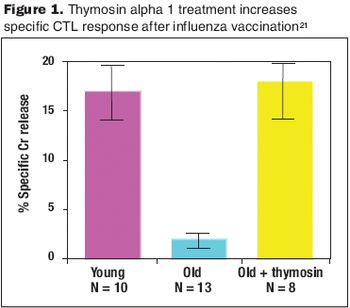
Clinical studies have shown that treatment with thymosin alpha 1 increases response to vaccination.
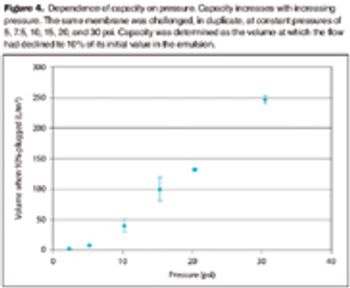
The viscosity of oily emulsions can reduce filter capacity and bacterial retention.
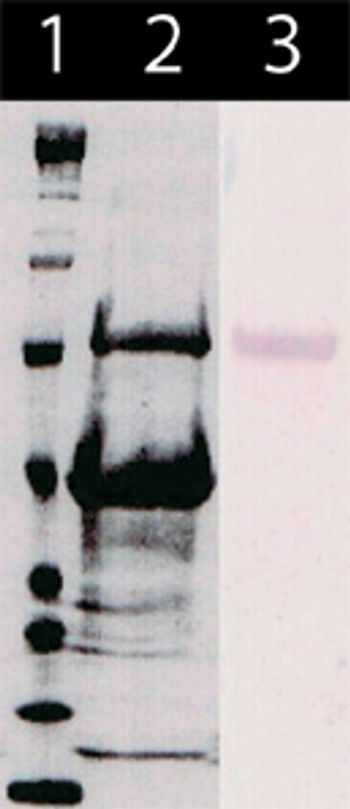
Recombinant vector technologies can improve the yield and lower the cost of egg-based influenza vaccine production.

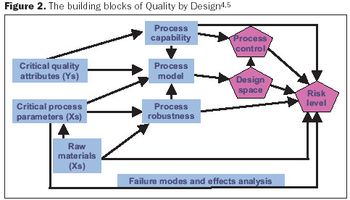
The nimbleness of biotechs makes them well suited to implementing QbD. Here's how to get started.
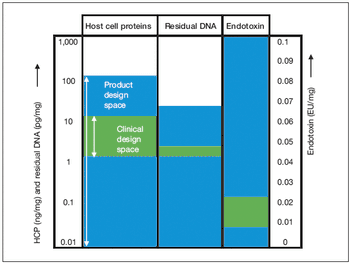
Key considerations for defining your overall control strategy.
