
Xiao-Ping Dai, PhD
Advertisement
Xiao-Ping Dai, PhD, manager at Cell Culture Science, Global Manufacturing and Supply, Bristol-Myers Squibb Company.
Articles by Xiao-Ping Dai, PhD

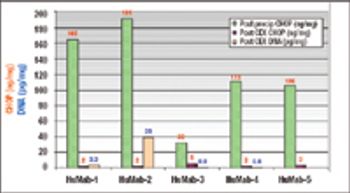
Precipitation prior to capture chromatography offers a simple, robust, and economical method to remove CHO host cell proteins and DNA.
Advertisement
Latest Updated Articles
 A Generic Growth Test Method for Improving Quality Control of Disposables in Industrial Cell Culture
A Generic Growth Test Method for Improving Quality Control of Disposables in Industrial Cell CulturePublished: June 1st 2013 | Updated:
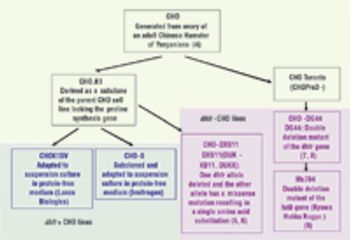
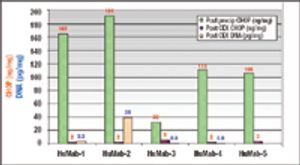 Precipitation of Process-Derived Impurities in Non-Protein A Purification Schemes for Antibodies
Precipitation of Process-Derived Impurities in Non-Protein A Purification Schemes for AntibodiesPublished: October 2nd 2009 | Updated:
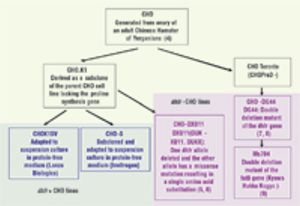 The Impact of Cell Culture Medium on Cell Line and Process Development Timelines and Strategies
The Impact of Cell Culture Medium on Cell Line and Process Development Timelines and StrategiesPublished: June 2nd 2009 | Updated:
Advertisement
Advertisement
Trending on BioPharm International
1
First-in-Human Study Validates Safety of Next-Generation mRNA–LNP Platform
2
FDA Clears PharmaResearch IND for Nano-Based Cancer Drug PRD-101
3
How CDMO Alliances Can Provide End-to-End Service that Reduces Drug Development Time and Costs
4
High-Dose Nusinersen Slows Neurodegeneration in SMA Patients, Study Shows
5
