Rolling-tube system balances scale-up accuracy and thoroughput.

Rolling-tube system balances scale-up accuracy and thoroughput.

Identify the best experimentation methods for the data you need.
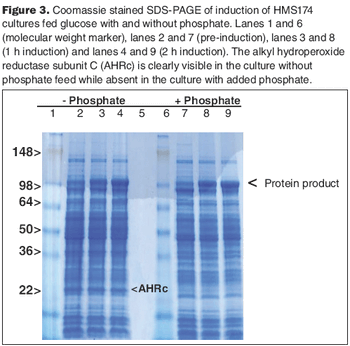
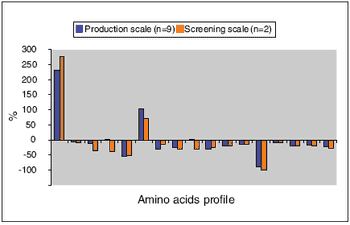
The HMS174 strain, in the absence or presence of excess phosphate, can metabolize acetate efficiently.

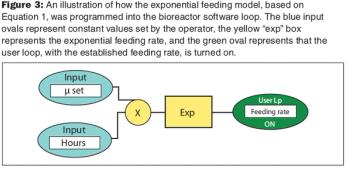
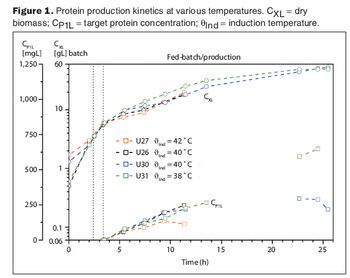
An exponential feeding strategy coupled with automation doubled protein yields while reducing fermentation time by 25%.
Two case studies show how advanced information technologies make process development more efficient.
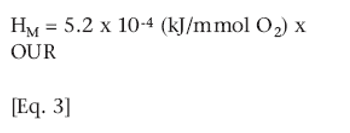
Wet testing of microbial fermenters allows for a greater understanding of the equipment's capabilities.

The quest for increased productivity and better process control combined with patient safety has encouraged biopharmaceutical companies to use chemically defined media for cell culture.

Plant-derived hydrolysates can be used as valuable and practical tools to improve cell culture performance.

Computational fluid dynamics can resolve performance problems.
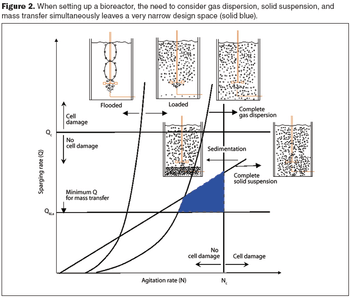
Computational fluid dynamics is a powerful tool to optimize processes.

Gaining a license can be a complex process, but a few key tips can help you avoid common pitfalls and patent infringements.

How to reduce plasmid-mediated metabolic burden for higher yields.
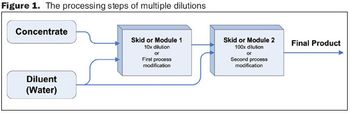
Automated in-line dilution can help solve capacity, financial, and quality concerns that biopharmaceutical manufacturing plants may be facing.

To assess current trends in fermentation and cell culture equipment, BioPharm International turned to Geoff Hodge, managing director of process technology, Xcellerex, Inc.; Günter Jagschies, senior director of strategic customer relations, life sciences, biotechnologies, GE Healthcare; and Rich Mirro, product manager, New Brunswick Scientific.
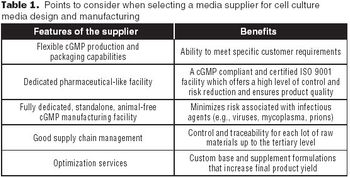
To select the right partner for media design and optimization services, several key factors must be considered.
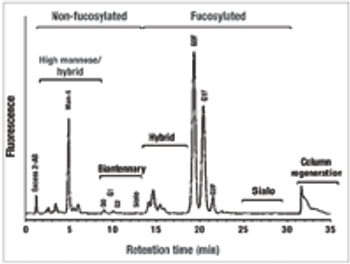
Select the best approach to determine critical quality attributes.

Companies often wait for a critical mass before adopting new technologies. But if no one takes the risk, critical mass will never be reached.
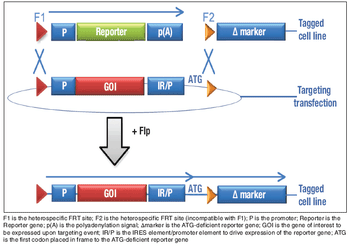
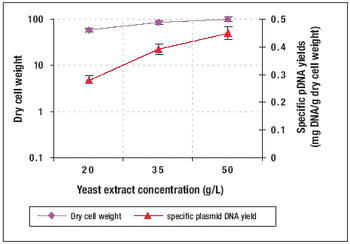
How to produce Plasmid DNA in a high-cell-density culture.
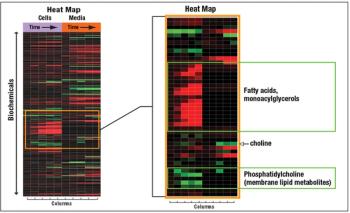
Through metabolomics, the metabolic underpinnings of cellular changes can be rapidly pinpointed, directing process development scientists to key areas for cell culture optimization.
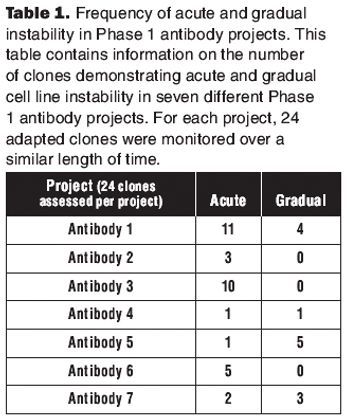
By considering stability as part of the cell line selection and cell banking paradigm, we can ensure that instability problems are not observed during clinical or commercial manufacturing.
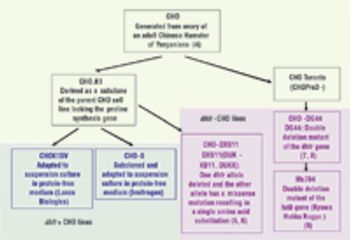
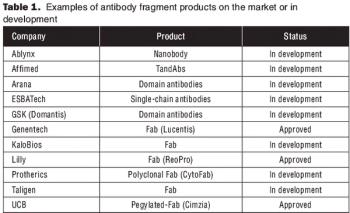
Microbial systems such as E. Coli and yeasts are most effective for producing antibody fragments.

A prove-free system monitors accurately at very small scale.